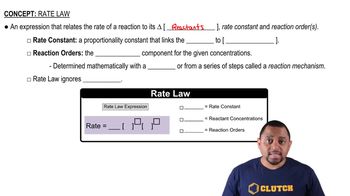
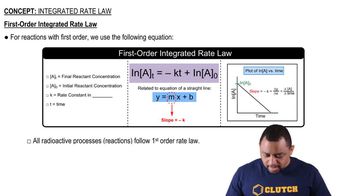
Consider the following reaction:
2 NO(g) + 2 H2(g) → N2(g) + 2 H2O(g)
(b) If the rate constant for this reaction at 1000 K is 6.0 × 104 M-2 s-1, what is the reaction rate when [NO] = 0.035 M and [H2] = 0.015 M?
(c) What is the reaction rate at 1000 K when the concentration of NO is increased to 0.10 M, while the concentration of H2 is 0.010 M?