The decomposition reaction of N2O5 in carbon tetrachloride is 2 N2O5 → 4 NO2 + O2. The rate law is first order in N2O5. At 64°C the rate constant is 4.82 × 10-3 s-1. (a) Write the rate law for the reaction.

Consider the following reaction: 2 NO1g2 + 2 H21g2¡N21g2 + 2 H2O1g2 (d) What is the reaction rate at 1000 K if [NO] is decreased to 0.010 M and 3H24 is increased to 0.030 M?
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
Reaction Rate

Concentration and Its Effect

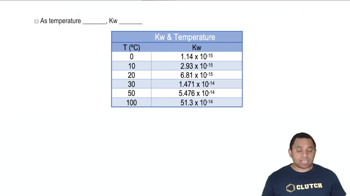
Temperature Dependence
The decomposition reaction of N2O5 in carbon tetrachloride is 2 N2O5 → 4 NO2 + O2. The rate law is first order in N2O5. At 64°C the rate constant is 4.82 × 10-3 s-1. (c) What happens to the rate when the concentration of N2O5 is doubled to 0.0480 M? (d) What happens to the rate when the concentration of N2O5 is halved to 0.0120 M?
Consider the following reaction:
2 NO(g) + 2 H2(g) → N2(g) + 2 H2O(g)
(b) If the rate constant for this reaction at 1000 K is 6.0 × 104 M-2 s-1, what is the reaction rate when [NO] = 0.035 M and [H2] = 0.015 M?
(c) What is the reaction rate at 1000 K when the concentration of NO is increased to 0.10 M, while the concentration of H2 is 0.010 M?
The reaction between ethyl bromide (C2H5Br) and hydroxide ion in ethyl alcohol at 330 K, C2H5Br(alc) + OH-(alc) → C2H5OH(l) + Br-(alc), is first order each in ethyl bromide and hydroxide ion. When [C2H5Br] is 0.0477 M and [OH-] is 0.100 M, the rate of disappearance of ethyl bromide is 1.7×10-7 M/s. (a) What is the value of the rate constant?
The reaction between ethyl bromide (C2H5Br) and hydroxide ion in ethyl alcohol at 330 K, C2H5Br(alc) + OH-(alc) → C2H5OH(l) + Br-(alc), is first order each in ethyl bromide and hydroxide ion. When [C2H5Br] is 0.0477 M and [OH-] is 0.100 M, the rate of disappearance of ethyl bromide is 1.7×10-7 M/s. (b) What are the units of the rate constant?
