The decomposition reaction of N2O5 in carbon tetrachloride is 2 N2O5 → 4 NO2 + O2. The rate law is first order in N2O5. At 64°C the rate constant is 4.82 × 10-3 s-1. (c) What happens to the rate when the concentration of N2O5 is doubled to 0.0480 M? (d) What happens to the rate when the concentration of N2O5 is halved to 0.0120 M?

Consider the following reaction: CH3Br(aq) + OH-(aq) → CH3OH(aq) + Br-(aq). The rate law for this reaction is first order in CH3Br and first order in OH-. When [CH3Br] is 5.0 * 10^-3 M and [OH-] is 0.050 M, the reaction rate at 298 K is 0.0432 M/s. (c) What would happen to the rate if the concentration of OH- were tripled? (d) What would happen to the rate if the concentration of both reactants were tripled?
 Verified step by step guidance
Verified step by step guidanceKey Concepts
Rate Law
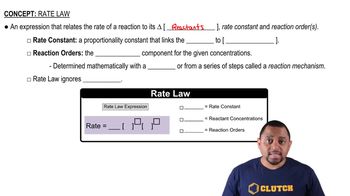
Order of Reaction

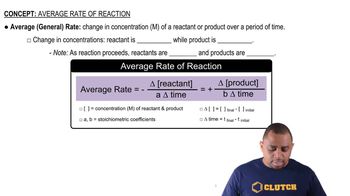
Concentration and Reaction Rate
Consider the following reaction:
2 NO(g) + 2 H2(g) → N2(g) + 2 H2O(g)
(b) If the rate constant for this reaction at 1000 K is 6.0 × 104 M-2 s-1, what is the reaction rate when [NO] = 0.035 M and [H2] = 0.015 M?
(c) What is the reaction rate at 1000 K when the concentration of NO is increased to 0.10 M, while the concentration of H2 is 0.010 M?
Consider the following reaction: 2 NO1g2 + 2 H21g2¡N21g2 + 2 H2O1g2 (d) What is the reaction rate at 1000 K if [NO] is decreased to 0.010 M and 3H24 is increased to 0.030 M?
The reaction between ethyl bromide (C2H5Br) and hydroxide ion in ethyl alcohol at 330 K, C2H5Br(alc) + OH-(alc) → C2H5OH(l) + Br-(alc), is first order each in ethyl bromide and hydroxide ion. When [C2H5Br] is 0.0477 M and [OH-] is 0.100 M, the rate of disappearance of ethyl bromide is 1.7×10-7 M/s. (a) What is the value of the rate constant?
The reaction between ethyl bromide (C2H5Br) and hydroxide ion in ethyl alcohol at 330 K, C2H5Br(alc) + OH-(alc) → C2H5OH(l) + Br-(alc), is first order each in ethyl bromide and hydroxide ion. When [C2H5Br] is 0.0477 M and [OH-] is 0.100 M, the rate of disappearance of ethyl bromide is 1.7×10-7 M/s. (b) What are the units of the rate constant?
The reaction between ethyl bromide (C2H5Br) and hydroxide ion in ethyl alcohol at 330 K, C2H5Br(alc) + OH-(alc) → C2H5OH(l) + Br-(alc), is first order each in ethyl bromide and hydroxide ion. When [C2H5Br] is 0.0477 M and [OH-] is 0.100 M, the rate of disappearance of ethyl bromide is 1.7×10-7 M/s. (c) How would the rate of disappearance of ethyl bromide change if the solution were diluted by adding an equal volume of pure ethyl alcohol to the solution?
