Many metallic catalysts, particularly the precious-metal ones, are often deposited as very thin films on a substance of high surface area per unit mass, such as alumina (Al2O3) or silica (SiO2). (b) How does the surface area affect the rate of reaction?

The enzyme carbonic anhydrase catalyzes the reaction CO2(g) + H2O(l) ↔ HCO3⁻(aq) + H⁺(aq). In water, without the enzyme, the reaction proceeds with a rate constant of 0.039 s⁻¹ at 25 _x001E_C. In the presence of the enzyme in water, the reaction proceeds with a rate constant of 1.0 * 10⁶ s⁻¹ at 25 _x001E_C. Assuming the collision factor is the same for both situations, calculate the difference in activation energies for the uncatalyzed versus enzyme-catalyzed reaction.
 Verified step by step guidance
Verified step by step guidanceKey Concepts
Activation Energy

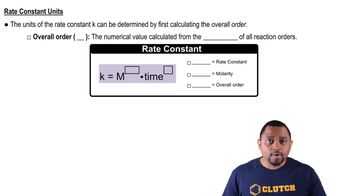
Rate Constant
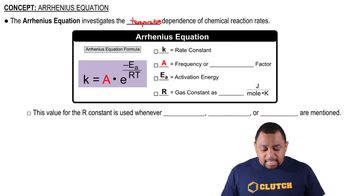
Arrhenius Equation
The enzyme urease catalyzes the reaction of urea, (NH2CONH2), with water to produce carbon dioxide and ammonia. In water, without the enzyme, the reaction proceeds with a first-order rate constant of 4.15 × 10-5 s-1 at 100°C. In the presence of the enzyme in water, the reaction proceeds with a rate constant of 3.4 × 104 s-1 at 21°C. (b) If the rate of the catalyzed reaction were the same at 100°C as it is at 21°C, what would be the difference in the activation energy between the catalyzed and uncatalyzed reactions?
The enzyme urease catalyzes the reaction of urea, (NH2CONH2), with water to produce carbon dioxide and ammonia. In water, without the enzyme, the reaction proceeds with a first-order rate constant of 4.15 × 10-5 s-1 at 100°C. In the presence of the enzyme in water, the reaction proceeds with a rate constant of 3.4 × 104 s-1 at 21°C. (c) In actuality, what would you expect for the rate of the catalyzed reaction at 100°C as compared to that at 21°C?
The activation energy of an uncatalyzed reaction is 95 kJ/mol. The addition of a catalyst lowers the activation energy to 55 kJ/mol. Assuming that the collision factor remains the same, by what factor will the catalyst increase the rate of the reaction at (a) 25 C
