The enzyme urease catalyzes the reaction of urea, (NH2CONH2), with water to produce carbon dioxide and ammonia. In water, without the enzyme, the reaction proceeds with a first-order rate constant of 4.15 × 10-5 s-1 at 100°C. In the presence of the enzyme in water, the reaction proceeds with a rate constant of 3.4 × 104 s-1 at 21°C. (b) If the rate of the catalyzed reaction were the same at 100°C as it is at 21°C, what would be the difference in the activation energy between the catalyzed and uncatalyzed reactions?
Ch.14 - Chemical Kinetics

Brown14th EditionChemistry: The Central ScienceISBN: 9780134414232Not the one you use?Change textbook
Chapter 14, Problem 87a
The activation energy of an uncatalyzed reaction is 95 kJ/mol. The addition of a catalyst lowers the activation energy to 55 kJ/mol. Assuming that the collision factor remains the same, by what factor will the catalyst increase the rate of the reaction at (a) 25 C
 Verified step by step guidance
Verified step by step guidance1
Identify the Arrhenius equation: \( k = A e^{-\frac{E_a}{RT}} \), where \( k \) is the rate constant, \( A \) is the pre-exponential factor, \( E_a \) is the activation energy, \( R \) is the gas constant, and \( T \) is the temperature in Kelvin.
Convert the temperature from Celsius to Kelvin: \( T = 25 + 273.15 \).
Calculate the rate constant for the uncatalyzed reaction using the activation energy of 95 kJ/mol: \( k_1 = A e^{-\frac{95000}{8.314 \times T}} \).
Calculate the rate constant for the catalyzed reaction using the activation energy of 55 kJ/mol: \( k_2 = A e^{-\frac{55000}{8.314 \times T}} \).
Determine the factor by which the catalyst increases the rate of the reaction by taking the ratio \( \frac{k_2}{k_1} \).

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Was this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Activation Energy
Activation energy is the minimum energy required for a chemical reaction to occur. It represents the energy barrier that reactants must overcome to transform into products. A higher activation energy means that fewer molecules have sufficient energy to react, resulting in a slower reaction rate. Conversely, lowering the activation energy, such as through the use of a catalyst, increases the likelihood of successful collisions between reactant molecules.
Recommended video:
Guided course
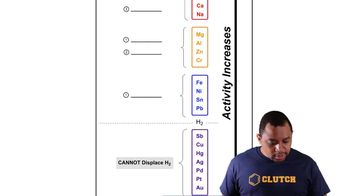
Activity Series Chart
Catalysts
Catalysts are substances that increase the rate of a chemical reaction without being consumed in the process. They work by providing an alternative reaction pathway with a lower activation energy. This allows more reactant molecules to have enough energy to undergo the reaction, thus speeding up the reaction rate. Importantly, catalysts do not alter the equilibrium position of the reaction; they only affect the rate at which equilibrium is reached.
Recommended video:
Guided course
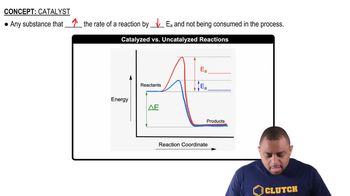
Catalyzed vs. Uncatalyzed Reactions
Arrhenius Equation
The Arrhenius equation describes the temperature dependence of reaction rates and relates the rate constant of a reaction to the activation energy and temperature. It is expressed as k = A * e^(-Ea/RT), where k is the rate constant, A is the pre-exponential factor, Ea is the activation energy, R is the universal gas constant, and T is the temperature in Kelvin. This equation illustrates how a decrease in activation energy, as seen with a catalyst, can significantly increase the reaction rate.
Recommended video:
Guided course
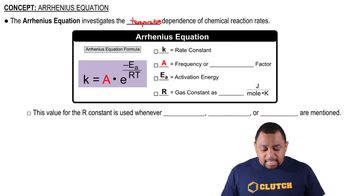
Arrhenius Equation
Related Practice
Textbook Question
Textbook Question
The enzyme urease catalyzes the reaction of urea, (NH2CONH2), with water to produce carbon dioxide and ammonia. In water, without the enzyme, the reaction proceeds with a first-order rate constant of 4.15 × 10-5 s-1 at 100°C. In the presence of the enzyme in water, the reaction proceeds with a rate constant of 3.4 × 104 s-1 at 21°C. (c) In actuality, what would you expect for the rate of the catalyzed reaction at 100°C as compared to that at 21°C?
Textbook Question
The activation energy of an uncatalyzed reaction is 95 kJ/mol. The addition of a catalyst lowers the activation energy to 55 kJ/mol. Assuming that the collision factor remains the same, by what factor will the catalyst increase the rate of the reaction at (b) 125 °C?
