Many metallic catalysts, particularly the precious-metal ones, are often deposited as very thin films on a substance of high surface area per unit mass, such as alumina (Al2O3) or silica (SiO2). (b) How does the surface area affect the rate of reaction?

(b) Automobile catalytic converters have to work at high temperatures, as hot exhaust gases stream through them. In what ways could this be an advantage? In what ways a disadvantage? (c) Why is the rate of flow of exhaust gases over a catalytic converter important?
 Verified step by step guidance
Verified step by step guidanceKey Concepts
Catalytic Converters
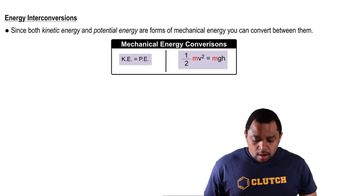
Temperature Effects on Reaction Rates
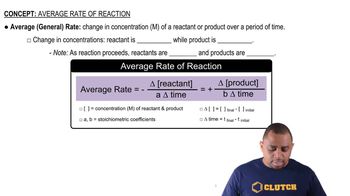
Flow Rate of Exhaust Gases
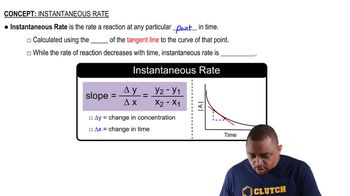
The enzyme urease catalyzes the reaction of urea, (NH2CONH2), with water to produce carbon dioxide and ammonia. In water, without the enzyme, the reaction proceeds with a first-order rate constant of 4.15 × 10-5 s-1 at 100°C. In the presence of the enzyme in water, the reaction proceeds with a rate constant of 3.4 × 104 s-1 at 21°C. (b) If the rate of the catalyzed reaction were the same at 100°C as it is at 21°C, what would be the difference in the activation energy between the catalyzed and uncatalyzed reactions?
The enzyme urease catalyzes the reaction of urea, (NH2CONH2), with water to produce carbon dioxide and ammonia. In water, without the enzyme, the reaction proceeds with a first-order rate constant of 4.15 × 10-5 s-1 at 100°C. In the presence of the enzyme in water, the reaction proceeds with a rate constant of 3.4 × 104 s-1 at 21°C. (c) In actuality, what would you expect for the rate of the catalyzed reaction at 100°C as compared to that at 21°C?
