Textbook Question
(a) Most commercial heterogeneous catalysts are extremely finely divided solid materials. Why is particle size important?

 Verified step by step guidance
Verified step by step guidance
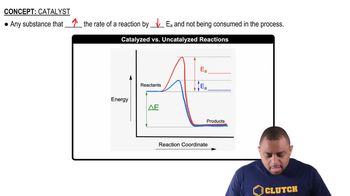
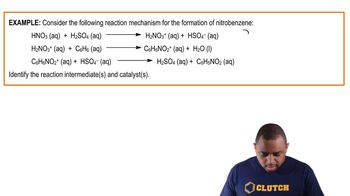

(a) Most commercial heterogeneous catalysts are extremely finely divided solid materials. Why is particle size important?
Many metallic catalysts, particularly the precious-metal ones, are often deposited as very thin films on a substance of high surface area per unit mass, such as alumina (Al2O3) or silica (SiO2). (b) How does the surface area affect the rate of reaction?