Textbook Question
(a) Most commercial heterogeneous catalysts are extremely finely divided solid materials. Why is particle size important?

 Verified step by step guidance
Verified step by step guidance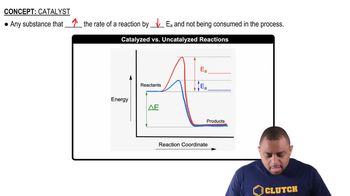


(a) Most commercial heterogeneous catalysts are extremely finely divided solid materials. Why is particle size important?
The addition of NO accelerates the decomposition of N2O, possibly by the following mechanism: NO1g2 + N2O1g2¡N21g2 + NO21g2 2 NO21g2¡2 NO1g2 + O21g2 (b) Is NO serving as a catalyst or an intermediate in this reaction?