The maximum allowable concentration of lead in drinking water is 9.0 ppb. (a) Calculate the molarity of lead in a 9.0-ppb solution.
Ch.13 - Properties of Solutions

Brown14th EditionChemistry: The Central ScienceISBN: 9780134414232Not the one you use?Change textbook
Chapter 13, Problem 97b
The maximum allowable concentration of lead in drinking water is 9.0 ppb. (b) How many grams of lead are in a swimming pool containing 9.0 ppb lead in 60 m3 of water?
 Verified step by step guidance
Verified step by step guidance1
Understand that 'ppb' stands for 'parts per billion', which means 9.0 ppb of lead means 9.0 grams of lead per 1 billion grams of water.
Calculate the mass of water in the swimming pool. Since the density of water is approximately 1 g/cm^3, convert the volume of the pool from cubic meters to grams. Note that 1 m^3 = 1,000,000 cm^3, so 60 m^3 = 60,000,000 cm^3, which is 60,000,000 grams of water.
Use the definition of ppb to find the mass of lead. Set up a proportion: (9.0 grams of lead / 1,000,000,000 grams of water) = (x grams of lead / 60,000,000 grams of water).
Solve the proportion for x to find the mass of lead in grams in the swimming pool.
Ensure the units are consistent and check the calculation for any errors.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
3mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Parts Per Billion (ppb)
Parts per billion (ppb) is a unit of measurement used to describe the concentration of a substance in a solution. It indicates how many parts of a substance are present in one billion parts of the total solution. In the context of drinking water, a concentration of 9.0 ppb means there are 9 grams of lead in one billion grams of water.
Recommended video:
Guided course
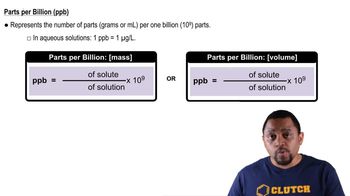
Parts per Billion (ppb)
Volume and Density of Water
The volume of water is crucial for calculating the total mass of the solution. In this case, 60 m³ of water is equivalent to 60,000 liters, and since the density of water is approximately 1 g/mL, this volume translates to about 60,000,000 grams of water. Understanding this relationship allows for accurate calculations of the mass of lead present in the water.
Recommended video:
Guided course
Density via Water Displacement
Mass Calculation from Concentration
To find the mass of lead in the swimming pool, one can use the concentration in ppb and the total mass of the water. The formula involves multiplying the concentration (in grams per billion grams) by the total mass of the water (in grams). This calculation provides the total mass of lead present, which is essential for determining compliance with safety standards.
Recommended video:
Guided course
Molar Mass Calculation Example
Related Practice
Textbook Question
1
views
Textbook Question
Acetonitrile (CH3CN) is a polar organic solvent that dissolves a wide range of solutes, including many salts. The density of a 1.80 M LiBr solution in acetonitrile is 0.826 g/cm3. Calculate the concentration of the solution in (a) molality,
Textbook Question
Acetonitrile (CH3CN) is a polar organic solvent that dissolves a wide range of solutes, including many salts. The density of a 1.80 M LiBr solution in acetonitrile is 0.826 g/cm3. Calculate the concentration of the solution in (b) mole fraction of LiBr,
1
views
Textbook Question
Acetonitrile (CH3CN) is a polar organic solvent that dissolves a wide range of solutes, including many salts. The density of a 1.80 M LiBr solution in acetonitrile is 0.826 g/cm3. Calculate the concentration of the solution in (c) mass percentage of CH3CN.
