Textbook Question
At three different temperatures, T1, T2, and T3, the molecules in a liquid crystal align in these ways:
(b) Which is the highest of these three temperatures? [Section 11.7]

 Verified step by step guidance
Verified step by step guidance


At three different temperatures, T1, T2, and T3, the molecules in a liquid crystal align in these ways:
(b) Which is the highest of these three temperatures? [Section 11.7]
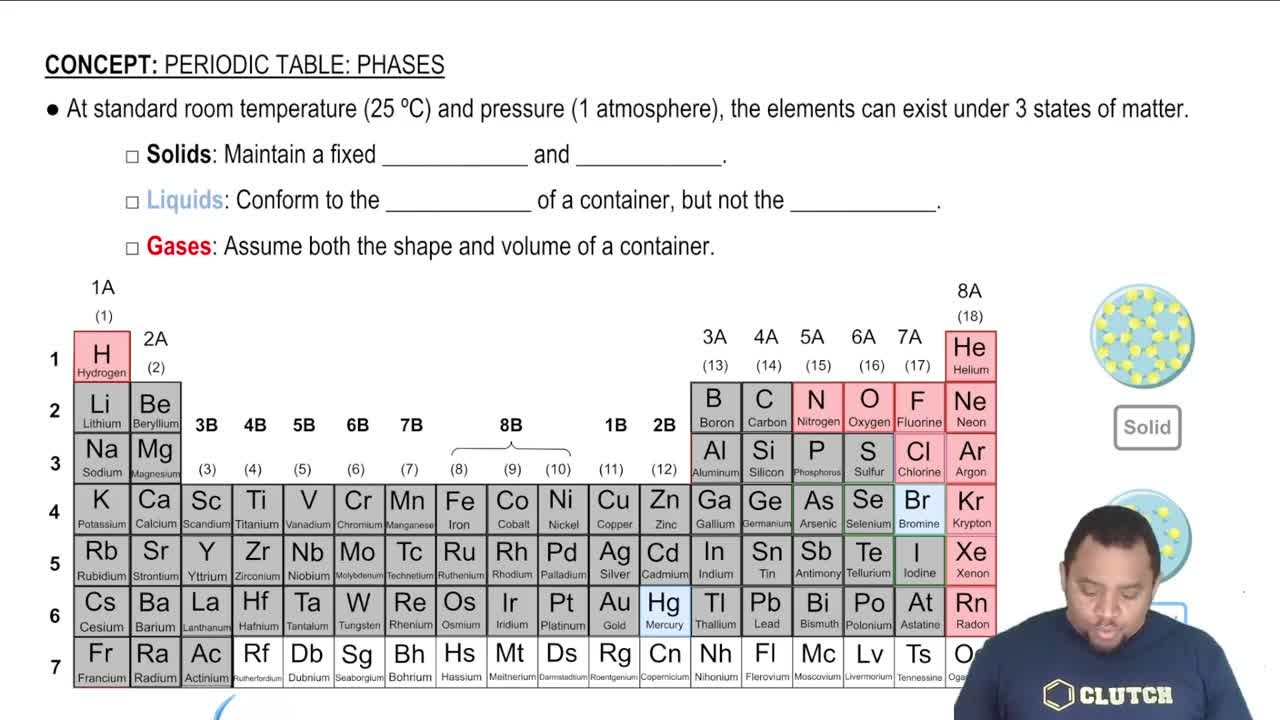
List the three states of matter in order of (a) increasing molecular disorder
(a) How does the average kinetic energy of molecules com- pare with the average energy of attraction between mole- cules in solids, liquids, and gases?
(c) What happens to a gas if you put it under extremely high pressure?