
Carbon dioxide, which is recognized as the major contributor to global warming as a 'greenhouse gas,' is formed when fossil fuels are combusted, as in electrical power plants fueled by coal, oil, or natural gas. One potential way to reduce the amount of CO2 added to the atmosphere is to store it as a compressed gas in underground formations. Consider a 1000-megawatt coal-fired power plant that produces about 6×106 tons of CO2 per year. (a) Assuming ideal-gas behavior, 101.3 kPa, and 27 °C, calculate the volume of CO2 produced by this power plant.
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
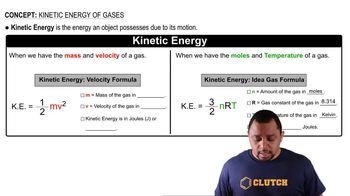
Ideal Gas Law

Greenhouse Gases
Combustion of Fossil Fuels

A gas bubble with a volume of 1.0 mm3 originates at the bottom of a lake where the pressure is 3.0 atm. Calculate its volume when the bubble reaches the surface of the lake where the pressure is 730 torr, assuming that the temperature does not change.
Carbon dioxide, which is recognized as the major contributor to global warming as a “greenhouse gas,” is formed when fossil fuels are combusted, as in electrical power plants fueled by coal, oil, or natural gas. One potential way to reduce the amount of CO2 added to the atmosphere is to store it as a compressed gas in underground formations. Consider a 1000-megawatt coal-fired power plant that produces about 6⨉106 tons of CO2 per year. (b) If the CO2 is stored underground as a liquid at 10 C and 12.16 MPa and a density of 1.2 g/cm3, what volume does it possess?
