Calculate the pressure that CCl4 will exert at 80 °C if 1.00 mol occupies 33.3 L, assuming that (a) CCl4 obeys the ideal-gas equation (b) CCl4 obeys the van der Waals equation. (Values for the van der Waals constants are given in Table 10.3.)

Torricelli, who invented the barometer, used mercury inits construction because mercury has a very high density,which makes it possible to make a more compact barometerthan one based on a less dense fluid. Calculate the densityof mercury using the observation that the column ofmercury is 760 mm high when the atmospheric pressure is1.01 * 105 Pa. Assume the tube containing the mercury isa cylinder with a constant cross-sectional area.
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
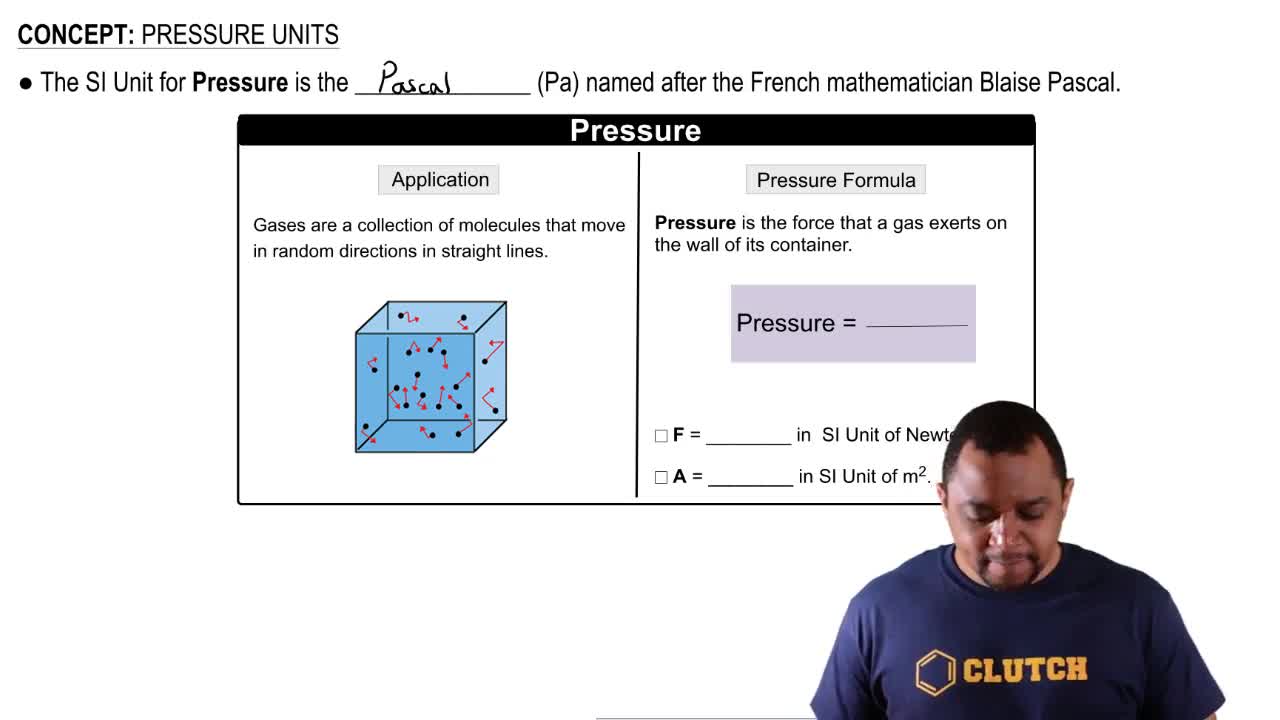
Key Concepts
Density

Atmospheric Pressure

Hydrostatic Pressure
Calculate the pressure that CCl4 will exert at 80 °C if 1.00 mol occupies 33.3 L, assuming that (c) Which would you expect to deviate more from ideal behavior under these conditions, Cl2 or CCl4? Explain.
Table 10.3 shows that the van der Waals b parameter has units of L/mol. This means that we can calculate the sizes of atoms or molecules from the b parameter. Refer back to the discussion in Section 7.3. Is the van der Waals radius we calculate from the b parameter of Table 10.3 more closely associated with the bonding or nonbonding atomic radius discussed there? Explain.
A gas bubble with a volume of 1.0 mm3 originates at the bottom of a lake where the pressure is 3.0 atm. Calculate its volume when the bubble reaches the surface of the lake where the pressure is 730 torr, assuming that the temperature does not change.
Carbon dioxide, which is recognized as the major contributor to global warming as a 'greenhouse gas,' is formed when fossil fuels are combusted, as in electrical power plants fueled by coal, oil, or natural gas. One potential way to reduce the amount of CO2 added to the atmosphere is to store it as a compressed gas in underground formations. Consider a 1000-megawatt coal-fired power plant that produces about 6×106 tons of CO2 per year. (a) Assuming ideal-gas behavior, 101.3 kPa, and 27 °C, calculate the volume of CO2 produced by this power plant.
