Textbook Question
(a) What is a functional group?

 Verified step by step guidance
Verified step by step guidance
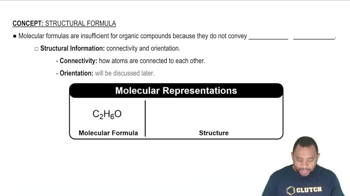
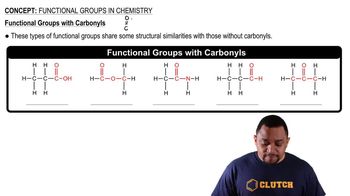
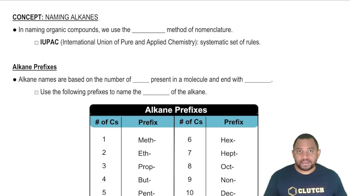
(a) What is a functional group?
(b) What functional group characterizes an alcohol?
(c) Write a structural formula for 1-pentanol, the alcohol derived from pentane by making a substitution on one of the carbon atoms.
Consider the following organic substances: ethylethanoate, ethylmethylether, hexanol, and propanone. (b) Which of these molecules contain a C = O group?
Chloropropane is derived from propane by substituting Cl for H on one of the carbon atoms. (a) Draw the structural formulas for the two isomers of chloropropane.
Chloropropane is derived from propane by substituting Cl for H on one of the carbon atoms. (b) Suggest names for these two compounds.