What is the general trend in standard potentials for the oxidation of first-series transition metals from Sc to Zn? What is the reason for the trend?
Ch.21 - Transition Elements and Coordination Chemistry

Chapter 21, Problem 21.8
Based on the wavelength of maximum absorption of the cobalt complexes, arrange the following ligands in a spectrochemical series from weakest-field to strongest-field ligand.
(a) Cl- < NCS- < H2O < NH3
(b) Cl- < NCS- < H2O < NH3
(c) H2O < Cl- < NH3 < NCS-
(d) Cl- < H2O < NCS- < NH3
 Verified step by step guidance
Verified step by step guidance1
Understand the concept of the spectrochemical series, which ranks ligands based on their ability to split the d-orbitals of a metal ion.
Recall that ligands causing a smaller splitting (weak-field ligands) result in longer wavelengths of maximum absorption, while those causing larger splitting (strong-field ligands) result in shorter wavelengths.
Identify the typical order of ligands in the spectrochemical series: Cl^- < H2O < NCS^- < NH3.
Compare the given options with the typical order to determine which one matches the weakest to strongest field ligands.
Select the option that correctly arranges the ligands from weakest-field to strongest-field based on the spectrochemical series.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Was this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Spectrochemical Series
The spectrochemical series is a ranking of ligands based on their ability to split the d-orbitals of transition metal complexes. Ligands that produce a larger splitting of the d-orbitals are considered strong-field ligands, while those that cause less splitting are weak-field ligands. This series helps predict the color and magnetic properties of the complexes formed with different ligands.
Recommended video:
Guided course

Activity Series Chart
Crystal Field Theory
Crystal Field Theory (CFT) explains the electronic structure of transition metal complexes by considering the interaction between the metal ion and surrounding ligands. According to CFT, the presence of ligands causes the degenerate d-orbitals to split into different energy levels, influencing the absorption of light and the resulting color of the complex. Understanding CFT is essential for analyzing how ligands affect the properties of metal complexes.
Recommended video:
Guided course

The study of ligand-metal interactions helped to form Ligand Field Theory which combines CFT with MO Theory.
Ligand Field Strength
Ligand field strength refers to the ability of a ligand to influence the energy levels of the d-orbitals in a transition metal complex. Strong-field ligands, such as NH3 and CN-, cause a greater splitting of the d-orbitals compared to weak-field ligands like Cl-. This concept is crucial for determining the arrangement of ligands in the spectrochemical series and predicting the behavior of the complexes.
Recommended video:
Guided course
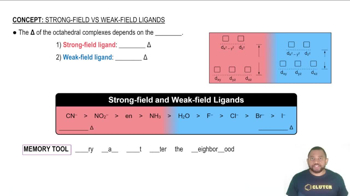
Strong-Field Ligands result in a large Δ and Weak-Field Ligands result in a small Δ.
Related Practice
Textbook Question
Textbook Question
What is the crystal field energy level diagram for the complex [Fe(NH3)6]3+?
(a)
(b)
(c)
(d)
Textbook Question
Predict the crystal field energy-level diagram for a linear ML2 complex that has two ligands along the :
Textbook Question
For each of the following complexes, draw a crystal field energy-level diagram, assign the electrons to orbitals, and predict the number of unpaired electrons.
(a) [CrF6]3-
(b) [V(H2O)6]3+
(c) [Fe(CN)6]3-
Textbook Question
Which of the following complexes are paramagnetic?
(a) [Mn(CN)6]3-
(b) [Zn(NH3)4]2+ (tetrahedral)
(c) [Fe(CN)6]4-
(d) [FeF6]4-
Textbook Question
Draw a crystal field energy-level diagram, assign the electrons to orbitals, and predict the number of unpaired electrons for each of the following.
(a) [Cu(en)3]2+
(b) [FeF6]2-
(c) [Co(en)3]3+ (low spin)
