The reaction A¡products was monitored as a function of time. The results are shown here. Time (s) [A] (M) 0 1.000 25 0.914 50 0.829 75 0.744 100 0.659 125 0.573 150 0.488 175 0.403 200 0.318 Determine the order of the reaction and the value of the rate constant. What is the rate of reaction when [A] = 0.10 M?

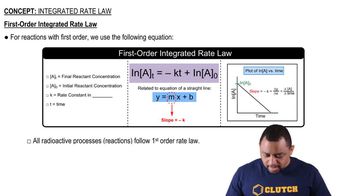
This reaction was monitored as a function of time: A → B + C A plot of ln[A] versus time yields a straight line with slope -0.0045/s. c. What is the half-life?
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
First-Order Reactions
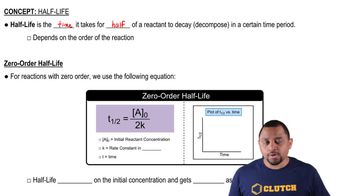
Half-Life
Rate Constant (k)

This reaction was monitored as a function of time: A → B + C A plot of ln[A] versus time yields a straight line with slope -0.0045/s. a. What is the value of the rate constant (k) for this reaction at this temperature?
This reaction was monitored as a function of time: A → B + C A plot of ln[A] versus time yields a straight line with slope -0.0045/s. b. Write the rate law for the reaction.
This reaction was monitored as a function of time: A → B + C A plot of ln[A] versus time yields a straight line with slope -0.0045/s. d. If the initial concentration of A is 0.250 M, what is the concentration after 225 s?
This reaction was monitored as a function of time: AB → A + B A plot of 1/[AB] versus time yields a straight line with a slope of +0.55/Ms.
a. What is the value of the rate constant (k) for this reaction at this temperature?
This reaction was monitored as a function of time: AB → A + B A plot of 1/[AB] versus time yields a straight line with a slope of +0.55/Ms. b. Write the rate law for the reaction.
