At 28 C, raw milk sours in 4.0 h but takes 48 h to sour in a refrigerator at 5 C. Estimate the activation energy in kJ>mol for the reaction that leads to the souring of milk.
Ch.14 - Chemical Kinetics

Brown14th EditionChemistry: The Central ScienceISBN: 9780134414232Not the one you use?Change textbook
Chapter 14, Problem 107a
The following mechanism has been proposed for the reaction of NO with H2 to form N2O and H2O:
NO(g) + NO(g) → N2O2(g)
N2O2(g) + H2(g) → N2O(g) + H2O(g)
(a) Show that the elementary reactions of the proposed mechanism add to provide a balanced equation for the reaction.
 Verified step by step guidance
Verified step by step guidance1
Step 1: Write down the two elementary reactions given in the mechanism. The first reaction is: NO(g) + NO(g) → N2O2(g). The second reaction is: N2O2(g) + H2(g) → N2O(g) + H2O(g).
Step 2: Add the reactants from both elementary reactions together. This gives: NO(g) + NO(g) + N2O2(g) + H2(g).
Step 3: Add the products from both elementary reactions together. This gives: N2O2(g) + N2O(g) + H2O(g).
Step 4: Cancel out any species that appear on both sides of the equation. In this case, N2O2(g) appears as both a reactant and a product, so it can be canceled out.
Step 5: Write the final balanced equation after canceling out common species. The balanced equation is: 2NO(g) + H2(g) → N2O(g) + H2O(g). This shows that the elementary reactions add up to provide the balanced equation for the overall reaction.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
2mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Elementary Reactions
Elementary reactions are the simplest types of chemical reactions that occur in a single step, involving a direct interaction between reactants to form products. Each elementary reaction has its own rate law, which can be derived from its molecularity, indicating how many molecules are involved in the reaction. Understanding these reactions is crucial for analyzing reaction mechanisms and determining the overall stoichiometry of a chemical process.
Recommended video:
Guided course
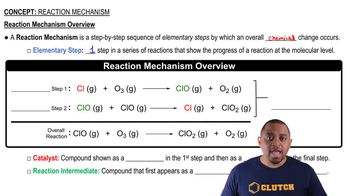
Reaction Mechanism Overview
Balancing Chemical Equations
Balancing chemical equations involves ensuring that the number of atoms of each element is the same on both the reactant and product sides of the equation. This is based on the law of conservation of mass, which states that matter cannot be created or destroyed in a chemical reaction. A balanced equation provides a clear representation of the reactants and products, allowing for accurate stoichiometric calculations.
Recommended video:
Guided course

Balancing Chemical Equations
Reaction Mechanisms
A reaction mechanism is a detailed description of the step-by-step process by which reactants are converted into products. It includes all elementary reactions involved, their sequence, and the intermediates formed during the reaction. Understanding the mechanism is essential for predicting the rate of reaction and the conditions under which the reaction occurs, as well as for deriving the overall balanced equation from the individual steps.
Recommended video:
Guided course

Reaction Mechanism Overview
Related Practice
Textbook Question
Textbook Question
The following mechanism has been proposed for the reaction of NO with H2 to form N2O and H2O:
NO(g) + NO(g) → N2O2(g)
N2O2(g) + H2(g) → N2O(g) + H2O(g)
(d) The observed rate law is rate = k[NO]2[H2]. If the proposed mechanism is correct, what can we conclude about the relative speeds of the first and second reactions?
Textbook Question
Ozone in the upper atmosphere can be destroyed by the following two-step mechanism:
Cl(g) + O3(g) → ClO(g) + O2(g)
ClO(g) + O(g) → Cl(g) + O2(g)
(a) What is the overall equation for this process?
Textbook Question
Ozone in the upper atmosphere can be destroyed by the following two-step mechanism:
Cl(g) + O3(g) → ClO(g) + O2(g)
ClO(g) + O(g) → Cl(g) + O2(g)
(b) What is the catalyst in the reaction?
