At 28 C, raw milk sours in 4.0 h but takes 48 h to sour in a refrigerator at 5 C. Estimate the activation energy in kJ>mol for the reaction that leads to the souring of milk.

The following is a quote from an article in the August 18, 1998, issue of The New York Times about the breakdown of cellulose and starch: “A drop of 18 degrees Fahrenheit [from 77 _x001E_F to 59 _x001E_F] lowers the reaction rate six times; a 36-degree drop [from 77 _x001E_F to 41 _x001E_F] produces a fortyfold decrease in the rate.” (b) Assuming the value of Ea calculated from the 36 _x001E_ drop and that the rate of breakdown is first order with a half-life at 25 _x001E_C of 2.7 yr, calculate the half-life for breakdown at a temperature of -15 _x001E_C.
 Verified step by step guidance
Verified step by step guidanceKey Concepts
Arrhenius Equation
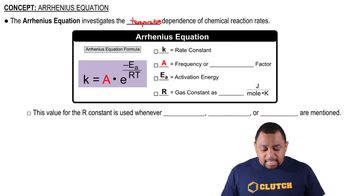
First-Order Reactions

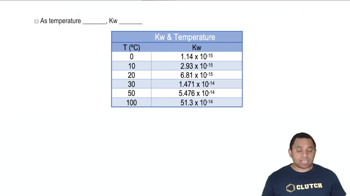
Temperature Dependence of Reaction Rates
The following mechanism has been proposed for the reaction of NO with H2 to form N2O and H2O:
NO(g) + NO(g) → N2O2(g)
N2O2(g) + H2(g) → N2O(g) + H2O(g)
(a) Show that the elementary reactions of the proposed mechanism add to provide a balanced equation for the reaction.
The following mechanism has been proposed for the reaction of NO with H2 to form N2O and H2O:
NO(g) + NO(g) → N2O2(g)
N2O2(g) + H2(g) → N2O(g) + H2O(g)
(d) The observed rate law is rate = k[NO]2[H2]. If the proposed mechanism is correct, what can we conclude about the relative speeds of the first and second reactions?
Ozone in the upper atmosphere can be destroyed by the following two-step mechanism:
Cl(g) + O3(g) → ClO(g) + O2(g)
ClO(g) + O(g) → Cl(g) + O2(g)
(a) What is the overall equation for this process?
