Assume that the MOs of diatomics from the third row of the periodic table, such as P2, are analogous to those from the second row.
a. Which valence atomic orbitals of P are used to construct the MOs of P2?

 Verified step by step guidance
Verified step by step guidance
Assume that the MOs of diatomics from the third row of the periodic table, such as P2, are analogous to those from the second row.
a. Which valence atomic orbitals of P are used to construct the MOs of P2?
Assume that the MOs of diatomics from the third row of the periodic table, such as P2, are analogous to those from the second row.
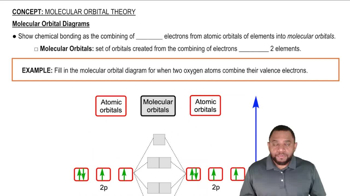
c. For the P2 molecule, how many electrons occupy the MO in the figure?
The iodine bromide molecule, IBr, is an interhalogen compound. Assume that the molecular orbitals of IBr are analogous to the homonuclear diatomic molecule F2. (a) Which valence atomic orbitals of I and of Br are used to construct the MOs of IBr?
An AB3 molecule is described as having a trigonal-bipyramidal electron-domain geometry. a. How many nonbonding domains are on atom A?
An AB3 molecule is described as having a trigonal-bipyramidal electron-domain geometry b. Based on the information given, which of the following is the molecular geometry of the molecule:
i. trigonal planar
ii. trigonal pyramidal
iii. T-shaped or
iv. tetrahedral?
Fill in the blank spaces in the following chart. If the molecule column is blank, find an example that fulfills the conditions of the rest of the row. Molecule Electron-Domain Hybridization Dipole Geometry of Central Atom Moment? Yes or No CO2 sp3 Yes sp3 No Trigonal planar No SF4 Octahedral No sp2 Yes Trigonal bipyramidal No XeF2