The iodine bromide molecule, IBr, is an interhalogen compound. Assume that the molecular orbitals of IBr are analogous to the homonuclear diatomic molecule F2. (c) One of the valence MOs of IBr is sketched here. Determine whether each of the following statements about this orbital is true: i. This is an antibonding orbital. ii. The larger contribution is from the I atom. iii. The energy of the molecular orbital is closer in energy to the valence atomic orbitals of Br than to those of I.
Ch.9 - Molecular Geometry and Bonding Theories

Brown15th EditionChemistry: The Central ScienceISBN: 9780137542970Not the one you use?Change textbook
Chapter 9, Problem 89
Fill in the blank spaces in the following chart. If the molecule column is blank, find an example that fulfills the conditions of the rest of the row. Molecule Electron-Domain Hybridization Dipole Geometry of Central Atom Moment? Yes or No CO2 sp3 Yes sp3 No Trigonal planar No SF4 Octahedral No sp2 Yes Trigonal bipyramidal No XeF2
 Verified step by step guidance
Verified step by step guidance1
Identify the missing information in the first row of the table: the molecule and the geometry of the central atom.
The given electron-domain hybridization is \( \text{sp}^3 \), which typically corresponds to a tetrahedral geometry.
The dipole moment is 'Yes', indicating that the molecule is polar.
A common molecule with \( \text{sp}^3 \) hybridization and a polar nature is \( \text{CH}_3\text{Cl} \) (chloromethane).
Thus, the first row can be completed with the molecule \( \text{CH}_3\text{Cl} \) and the geometry of the central atom as tetrahedral.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
1mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Electron-Domain Hybridization
Electron-domain hybridization is the process by which atomic orbitals mix to form new hybrid orbitals that can accommodate the electron pairs around a central atom. The type of hybridization (e.g., sp, sp2, sp3) depends on the number of electron domains, which include bonding pairs and lone pairs. Understanding this concept is crucial for predicting molecular geometry and the arrangement of atoms in a molecule.
Recommended video:
Guided course
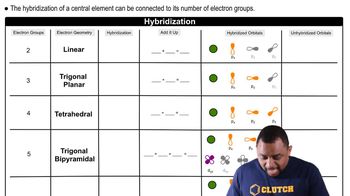
Hybridization and Electron Geometry
Molecular Geometry
Molecular geometry refers to the three-dimensional arrangement of atoms in a molecule, which is determined by the repulsion between electron pairs surrounding the central atom. The VSEPR (Valence Shell Electron Pair Repulsion) theory helps predict the geometry based on the number of bonding and lone pairs. This concept is essential for understanding the shape and polarity of molecules, which influences their chemical behavior.
Recommended video:
Guided course

Molecular Geometry with Two Electron Groups
Dipole Moment
A dipole moment is a measure of the separation of positive and negative charges in a molecule, indicating its polarity. It arises when there is an uneven distribution of electron density, often due to differences in electronegativity between bonded atoms. Understanding dipole moments is important for predicting molecular interactions, solubility, and reactivity, as polar molecules behave differently than nonpolar ones.
Recommended video:
Guided course
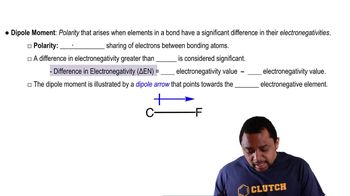
Dipole Moment
Related Practice
Textbook Question
Textbook Question
An AB3 molecule is described as having a trigonal-bipyramidal electron-domain geometry. a. How many nonbonding domains are on atom A?
Textbook Question
An AB3 molecule is described as having a trigonal-bipyramidal electron-domain geometry b. Based on the information given, which of the following is the molecular geometry of the molecule:
i. trigonal planar
ii. trigonal pyramidal
iii. T-shaped or
iv. tetrahedral?
Textbook Question
The lactic acid molecule, CH3CH(OH)COOH, gives sour milk its unpleasant, sour taste. e. What are the approximate bond angles around each carbon atom in the molecule?
Textbook Question
An AB5 molecule adopts the geometry shown here. b. What is the electron-domain geometry for the molecule?
