At standard temperature and pressure, the molar volumes of Cl2 and NH3 gases are 22.06 and 22.40 L, respectively (b) On cooling to 160 K, both substances form crystalline solids. Do you expect the molar volumes to decrease or increase on cooling the gases to 160 K?
Ch.11 - Liquids and Intermolecular Forces

Brown14th EditionChemistry: The Central ScienceISBN: 9780134414232Not the one you use?Change textbook
Chapter 11, Problem 13d
At standard temperature and pressure, the molar volumes of Cl2 and NH3 gases are 22.06 and 22.40 L, respectively (d) Are the molar volumes in the solid state as similar as they are in the gaseous state? Explain.
 Verified step by step guidance
Verified step by step guidance1
Step 1: Understand the concept of molar volume. The molar volume of a gas is the volume occupied by one mole of that gas at a certain temperature and pressure. In this case, we are given the molar volumes of Cl2 and NH3 gases at standard temperature and pressure (STP).
Step 2: Recognize that the molar volumes of gases are similar at STP due to the ideal gas law, which states that one mole of any gas at STP occupies a volume of approximately 22.4 L. This is because gases are mostly empty space, and the size of the individual gas molecules doesn't significantly affect the overall volume.
Step 3: Understand that the situation is different for solids. In the solid state, the particles are closely packed together, and the volume is greatly influenced by the size and shape of the individual particles. Therefore, the molar volumes of solids are not as similar as they are in the gaseous state.
Step 4: Consider the specific substances in question. Cl2 and NH3 have different sizes and shapes of molecules, and they form different types of crystal structures in the solid state. Therefore, their molar volumes in the solid state would not be as similar as in the gaseous state.
Step 5: Conclude that the molar volumes in the solid state are not as similar as they are in the gaseous state due to the differences in particle size, shape, and crystal structure.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
2mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Molar Volume
Molar volume is the volume occupied by one mole of a substance at a given temperature and pressure, typically measured in liters per mole (L/mol). At standard temperature and pressure (STP), gases have a molar volume of approximately 22.4 L/mol. This concept is crucial for comparing the behavior of different substances in gaseous and solid states, as it reflects how much space a mole of particles occupies.
Recommended video:
Guided course
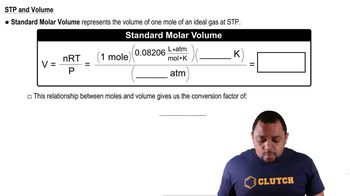
Standard Molar Volume
States of Matter
The states of matter—solid, liquid, and gas—are defined by the arrangement and energy of particles. In solids, particles are closely packed in a fixed arrangement, leading to a much lower molar volume compared to gases, where particles are far apart and move freely. Understanding these states helps explain why molar volumes differ significantly between gases and solids, as the intermolecular forces and particle spacing vary greatly.
Recommended video:
Guided course
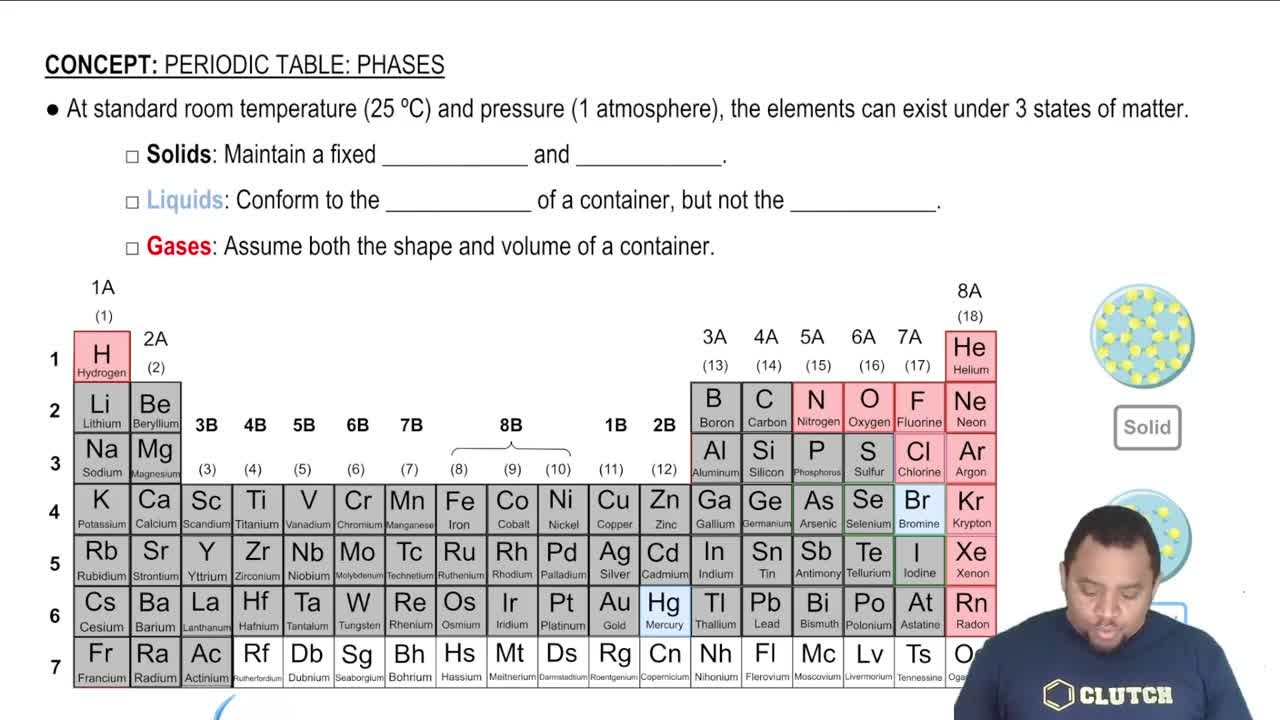
Element States of Matter
Intermolecular Forces
Intermolecular forces are the forces of attraction or repulsion between molecules, which significantly influence the physical properties of substances. In solids, strong intermolecular forces keep particles tightly packed, resulting in a small molar volume. In contrast, gases experience weaker forces, allowing for greater separation and larger molar volumes. Analyzing these forces is essential for understanding the differences in molar volumes between the gaseous and solid states.
Recommended video:
Guided course

Intermolecular vs Intramolecular Forces
Related Practice
Textbook Question
Textbook Question
At standard temperature and pressure, the molar volumes of Cl2 and NH3 gases are 22.06 and 22.40 L, respectively. (c) The densities of crystalline Cl2 and NH3 at 160 K are 2.02 and 0.84 g/cm3, respectively. Calculate their molar volumes.
Textbook Question
Benzoic acid, C6H5COOH, melts at 122 °C. The density in the liquid state at 130 °C is 1.08 g/cm3. The density of solid benzoic acid at 15 °C is 1.266 g/cm3. (b) If you converted a cubic centimeter of liquid benzoic acid into a solid, would the solid take up more, or less, volume than the original cubic centimeter of liquid?
1
views
Textbook Question
(a) Which type of intermolecular attractive force operates between all molecules?
3
views
