Textbook Question
At room temperature, Si is a solid, CCl4 is a liquid, and Ar is a gas. List these substances in order of (a) increasing intermolecular energy of attraction
3
views

 Verified step by step guidance
Verified step by step guidance


At room temperature, Si is a solid, CCl4 is a liquid, and Ar is a gas. List these substances in order of (a) increasing intermolecular energy of attraction
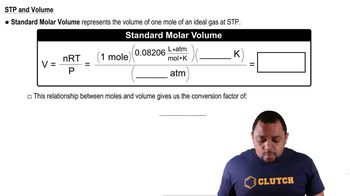
At standard temperature and pressure, the molar volumes of Cl2 and NH3 gases are 22.06 and 22.40 L, respectively. (c) The densities of crystalline Cl2 and NH3 at 160 K are 2.02 and 0.84 g/cm3, respectively. Calculate their molar volumes.
At standard temperature and pressure, the molar volumes of Cl2 and NH3 gases are 22.06 and 22.40 L, respectively (d) Are the molar volumes in the solid state as similar as they are in the gaseous state? Explain.