At standard temperature and pressure, the molar volumes of Cl2 and NH3 gases are 22.06 and 22.40 L, respectively (d) Are the molar volumes in the solid state as similar as they are in the gaseous state? Explain.
Ch.11 - Liquids and Intermolecular Forces

Brown14th EditionChemistry: The Central ScienceISBN: 9780134414232Not the one you use?Change textbook
Chapter 11, Problem 15a
(a) Which type of intermolecular attractive force operates between all molecules?
 Verified step by step guidance
Verified step by step guidance1
1. Intermolecular forces are the forces of attraction that exist between molecules. They are responsible for many physical properties of substances, such as boiling and melting points, phase changes, and the physical state of a substance.
2. There are several types of intermolecular forces, including dipole-dipole interactions, hydrogen bonding, and London dispersion forces.
3. Dipole-dipole interactions occur between polar molecules, which have a positive end and a negative end due to uneven distribution of electrons. Hydrogen bonding is a special type of dipole-dipole interaction that occurs when a hydrogen atom is bonded to a highly electronegative atom (like nitrogen, oxygen, or fluorine) and is attracted to another electronegative atom.
4. London dispersion forces, also known as van der Waals forces, are the weakest type of intermolecular force and occur between all molecules, whether they are polar or nonpolar. These forces arise due to temporary fluctuations in the electron distribution within a molecule, which create a temporary dipole that induces a dipole in a neighboring molecule.
5. Therefore, the type of intermolecular attractive force that operates between all molecules, regardless of their polarity or lack thereof, is the London dispersion force.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
2mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Intermolecular Forces
Intermolecular forces are the attractive forces that occur between molecules, influencing their physical properties such as boiling and melting points. These forces are generally weaker than intramolecular forces, which hold atoms together within a molecule. Understanding these forces is crucial for predicting how substances behave in different states of matter.
Recommended video:
Guided course

Intermolecular vs Intramolecular Forces
London Dispersion Forces
London dispersion forces, also known as van der Waals forces, are a type of intermolecular force that arises from temporary fluctuations in electron distribution within molecules. These forces are present in all molecules, regardless of polarity, and are particularly significant in nonpolar substances. They increase with the size and shape of the molecules involved.
Recommended video:
Guided course
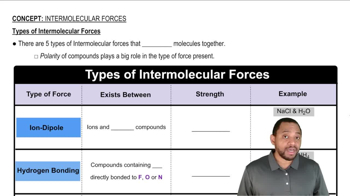
Types of Intermolecular Forces
Polarity and Dipole-Dipole Interactions
Polarity refers to the distribution of electrical charge over the atoms in a molecule, leading to regions of partial positive and negative charge. Dipole-dipole interactions occur between polar molecules, where the positive end of one molecule is attracted to the negative end of another. While these forces are stronger than London dispersion forces, they are not present in nonpolar molecules.
Recommended video:
Guided course
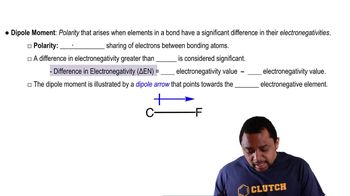
Dipole Moment
Related Practice
Textbook Question
1
views
Textbook Question
Benzoic acid, C6H5COOH, melts at 122 °C. The density in the liquid state at 130 °C is 1.08 g/cm3. The density of solid benzoic acid at 15 °C is 1.266 g/cm3. (b) If you converted a cubic centimeter of liquid benzoic acid into a solid, would the solid take up more, or less, volume than the original cubic centimeter of liquid?
1
views
Textbook Question
(b) Which type of intermolecular attractive force operates only between polar molecules?
3
views
Textbook Question
(c) Which type of intermolecular attractive force operates only between the hydrogen atom of a polar bond and a nearby small electronegative atom?
3
views
