Mass-Energy Equivalence
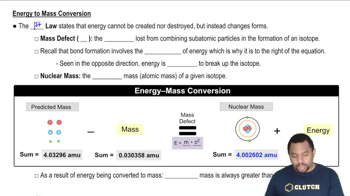
Mass-energy equivalence, expressed by Einstein's equation E=mc², indicates that mass can be converted into energy and vice versa. In nuclear reactions, such as the formation of nitrogen-16 from oxygen capturing a neutron, a small amount of mass is converted into energy, which can affect the stability and decay of the resulting isotope. This principle underlies the processes occurring in nuclear reactors.

 Verified step by step guidance
Verified step by step guidance