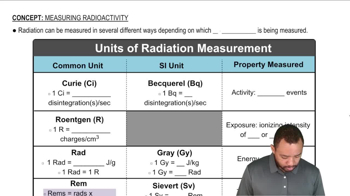
Textbook Question
The maximum level of radon in drinking water is 4.0 pCi (4.0 x 10^-12Ci) per milliliter. (a) How many disintegrations occur per minute in 1 mL of water at the maximum radon level? (b) If the radioactive isotope is 222Rn(t1/2 = 3.8 days), how many 222Rn atoms are present in 1 mL of the water?