Textbook Question
Balance the following equations and indicate whether they are combination, decomposition, or combustion reactions: (b) NH4NO3(s) → N2O(g) + H2O(g) (c) Zn(OH)2(s) → ZnO(s) + H2O(l) (d) Ag2O(s) → Ag(s) + O2(g)
2
views

 Verified step by step guidance
Verified step by step guidance
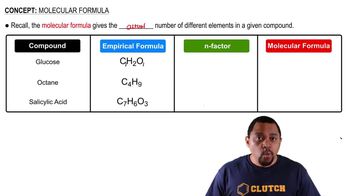

Balance the following equations and indicate whether they are combination, decomposition, or combustion reactions: (b) NH4NO3(s) → N2O(g) + H2O(g) (c) Zn(OH)2(s) → ZnO(s) + H2O(l) (d) Ag2O(s) → Ag(s) + O2(g)
Balance the following equations and indicate whether they are combination, decomposition, or combustion reactions: a. PbCO3(s) → PbO(s) + CO2(g)
Calculate the percentage by mass of oxygen in the following compounds: a. morphine, C17H19NO3
Calculate the percentage by mass of oxygen in the following compounds: b. codeine, C18H21NO3
Calculate the percentage by mass of oxygen in the following compounds: c. cocaine, C17H21NO4