Write a balanced chemical equation for the reaction that occurs when (a) titanium metal reacts with O21g2
Ch.3 - Chemical Reactions and Reaction Stoichiometry

Brown15th EditionChemistry: The Central ScienceISBN: 9780137542970Not the one you use?Change textbook
Chapter 3, Problem 21b,c,d
Balance the following equations and indicate whether they are combination, decomposition, or combustion reactions: (b) NH4NO3(s) → N2O(g) + H2O(g) (c) Zn(OH)2(s) → ZnO(s) + H2O(l) (d) Ag2O(s) → Ag(s) + O2(g)
 Verified step by step guidance
Verified step by step guidance1
Identify the type of reaction: This is a decomposition reaction because a single compound, NH_4NO_3, breaks down into two products, N_2O and H_2O.
Write the unbalanced chemical equation: NH_4NO_3(s) → N_2O(g) + H_2O(g).
List the number of each type of atom on both sides of the equation to see what needs balancing: On the left, there are 2 N, 4 H, and 3 O atoms. On the right, there are 2 N, 2 H, and 2 O atoms.
Balance the hydrogen atoms by adjusting the coefficient of H_2O: Since there are 4 H atoms on the left, place a coefficient of 2 in front of H_2O to balance the hydrogen atoms.
Check the balance of all atoms: After balancing hydrogen, ensure that the number of nitrogen and oxygen atoms are also balanced. Adjust coefficients if necessary to achieve balance for all elements.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
2mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Balancing Chemical Equations
Balancing chemical equations involves ensuring that the number of atoms of each element is the same on both sides of the equation. This is based on the law of conservation of mass, which states that matter cannot be created or destroyed in a chemical reaction. Balancing is crucial for accurately representing the reaction and predicting the amounts of reactants and products.
Recommended video:
Guided course

Balancing Chemical Equations
Types of Chemical Reactions
Chemical reactions can be classified into several types, including combination, decomposition, and combustion reactions. A combination reaction involves two or more substances combining to form a single product, while a decomposition reaction involves a single compound breaking down into two or more products. Combustion reactions typically involve a substance reacting with oxygen, producing heat and light.
Recommended video:
Guided course
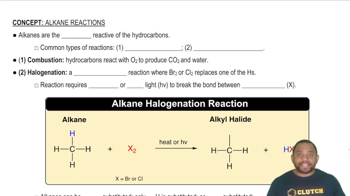
Common Types of Alkane Reactions
Decomposition Reactions
Decomposition reactions occur when a single compound breaks down into two or more simpler substances, often due to heat, light, or electricity. In the given equation, ammonium nitrate (NH4NO3) decomposes into nitrous oxide (N2O) and water (H2O). Recognizing this type of reaction is essential for classifying the reaction correctly and understanding the underlying chemical processes.
Recommended video:
Guided course
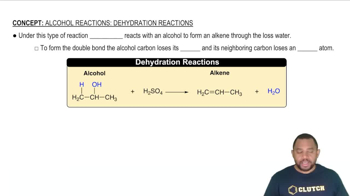
Alcohol Reactions: Dehydration Reactions
Related Practice
Textbook Question
1
views
Textbook Question
Write a balanced chemical equation for the reaction that occurs when (b) silver(I) oxide decomposes into silver metal and oxygen gas when heated (c) propanol, C3H7OH(l) burns in air (d) methyl tert-butyl ether, C5H12O(l), burns in air.
Textbook Question
Balance the following equations and indicate whether they are combination, decomposition, or combustion reactions: (a) C3H6(s) + O2(g) → CO2(g) + H2O(l)
2
views
Textbook Question
Balance the following equations and indicate whether they are combination, decomposition, or combustion reactions: a. PbCO3(s) → PbO(s) + CO2(g)
Textbook Question
Determine the formula weights of each of the following compounds: e. benzaldehyde, C6H5CHO, the molecule largely responsible for the odor of almond extract.
