Textbook Question
Write a balanced chemical equation for the reaction that occurs when (a) titanium metal reacts with O21g2
1
views

 Verified step by step guidance
Verified step by step guidance

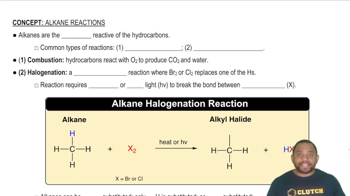
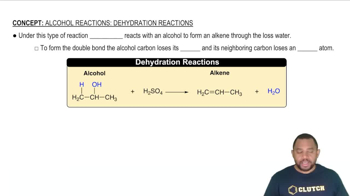
Write a balanced chemical equation for the reaction that occurs when (a) titanium metal reacts with O21g2
Balance the following equations and indicate whether they are combination, decomposition, or combustion reactions: (a) C3H6(s) + O2(g) → CO2(g) + H2O(l)
Balance the following equations and indicate whether they are combination, decomposition, or combustion reactions: (b) NH4NO3(s) → N2O(g) + H2O(g) (c) Zn(OH)2(s) → ZnO(s) + H2O(l) (d) Ag2O(s) → Ag(s) + O2(g)
Determine the formula weights of each of the following compounds: e. benzaldehyde, C6H5CHO, the molecule largely responsible for the odor of almond extract.
Calculate the percentage by mass of oxygen in the following compounds: a. morphine, C17H19NO3