An AB5 molecule adopts the geometry shown here.
c. Suppose the B atoms are halogen atoms. Of which group in the periodic table is atom A a member:
i. group 5A
ii. group 6A
iii. group 7A
iv. group 8A, or
v. is more information needed?

 Verified step by step guidance
Verified step by step guidance
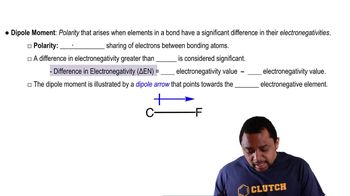
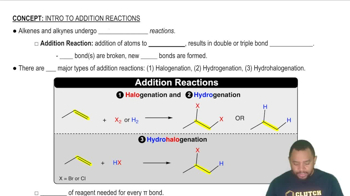
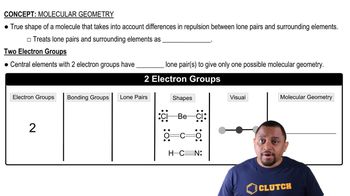
An AB5 molecule adopts the geometry shown here.
c. Suppose the B atoms are halogen atoms. Of which group in the periodic table is atom A a member:
i. group 5A
ii. group 6A
iii. group 7A
iv. group 8A, or
v. is more information needed?
The lactic acid molecule, CH3CH(OH)COOH, gives sour milk its unpleasant, sour taste. e. What are the approximate bond angles around each carbon atom in the molecule?
An AB5 molecule adopts the geometry shown here. b. What is the electron-domain geometry for the molecule?
a. Predict the electron-domain geometry around the central Xe atom in XeF2, XeF4, and XeF6.
Which of the following statements about hybrid orbitals is or are true? a. After an atom undergoes sp hybridization, there is one unhybridized p orbital on the atom, b. Under 𝑠𝑝2 hybridization, the large lobes point to the vertices of an equilateral triangle, and c. The angle between the large lobes of 𝑠𝑝3 hybrids is 109.5°.
The molecule C4H5N has the connectivity shown here. a. After the Lewis structure for the molecule is completed, how many 𝜎 and how many 𝜋 bonds are there in this molecule?