An AB5 molecule adopts the geometry shown here.
c. Suppose the B atoms are halogen atoms. Of which group in the periodic table is atom A a member:
i. group 5A
ii. group 6A
iii. group 7A
iv. group 8A, or
v. is more information needed?

 Verified step by step guidance
Verified step by step guidance

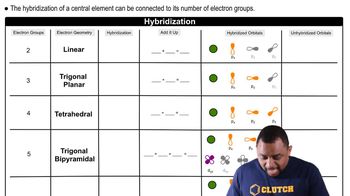

An AB5 molecule adopts the geometry shown here.
c. Suppose the B atoms are halogen atoms. Of which group in the periodic table is atom A a member:
i. group 5A
ii. group 6A
iii. group 7A
iv. group 8A, or
v. is more information needed?
The O—H bond lengths in the water molecule (H2O) are 0.96 Å, and the H—O—H angle is 104.5°. The overall dipole moment of the water molecule is 1.85 D. b. Calculate the magnitude of the bond dipole of the O─H bonds. (Note: You will need to use vector addition to do this.)
a. Predict the electron-domain geometry around the central Xe atom in XeF2, XeF4, and XeF6.
The molecule C4H5N has the connectivity shown here. a. After the Lewis structure for the molecule is completed, how many 𝜎 and how many 𝜋 bonds are there in this molecule?
Sodium azide is a shock-sensitive compound that releases N2 upon physical impact. The compound is used in automobile airbags. The azide ion is N3-. (a) Draw the Lewis structure of the azide ion that minimizes formal charge (it does not form a triangle). Is it linear or bent?
Sodium azide is a shock-sensitive compound that releases N2 upon physical impact. The compound is used in automobile airbags. The azide ion is N3-. (b) State the hybridization of the central N atom in the azide ion.