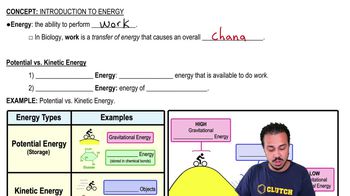
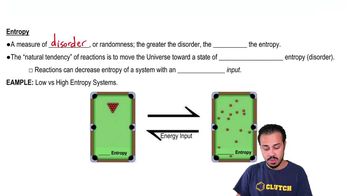
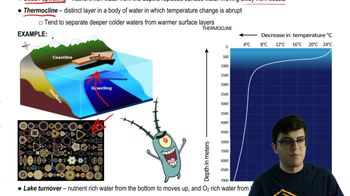
Which of the following occurs when a covalent bond forms?
a. Electrons in valence shells are transferred from one atom to another.
b. Electrons in valence shells are shared between atoms.
c. Partial charges on polar molecules interact.

 Verified step by step guidance
Verified step by step guidance
Which of the following occurs when a covalent bond forms?
a. Electrons in valence shells are transferred from one atom to another.
b. Electrons in valence shells are shared between atoms.
c. Partial charges on polar molecules interact.
What are the defining characteristics of a condensation reaction?
a. Two monomers are covalently bonded together and a water molecule is produced.
b. Two monomers are covalently bonded together and a water molecule is used up.
c. A polymer is broken down into monomers and a water molecule is produced.
d. A polymer is broken down into monomers and a water molecule is used up.
Which of these functional groups is known to be used for storing large amounts of chemical energy?
a. Amino group
b. Carbonyl group
c. Phosphate group
d. Sulfhydryl group
Which of these molecules would you predict to have the largest number of polar covalent bonds based on their molecular formulas?
a. C2H6O(ethanol)
b. C2H6(ethane)
c. C2H4O2(acetic acid)
d. C3H8O (propanol)
Locate fluorine (F) on the partial periodic table provided in Figure 2.2. Predict its relative electronegativity compared to hydrogen, sodium, and oxygen. State the number and type of bond(s) you expect it would form if it reacted with sodium (Na).
If you were given a solution that has a pH of 8.5, what would be its concentration of protons? What is the difference in proton concentration between this solution and one that has a pH of 7?