Back
BackProblem 80c
Use Rutherford's gold-foil experiment to answer each of the following:
c. How did he use the results to propose a model of the atom?
Problem 82b
Match the subatomic particles (1 to 3) to each of the descriptions below:
1. protons
2. neutrons
3. electrons
b. surround the nucleus
Problem 83a
Consider the following atoms in which X represents the chemical symbol of the element:
168X 169X 1810X 178X 188X
a. What atoms have the same number of protons?
Problem 83c
Consider the following atoms in which X represents the chemical symbol of the element:
168X 169X 1810X 178X 188X
c. Which atoms have the same mass number?
Problem 83d
Consider the following atoms in which X represents the chemical symbol of the element:
168X 169X 1810X 178X 188X
d. What atoms have the same number of neutrons?
Problem 85
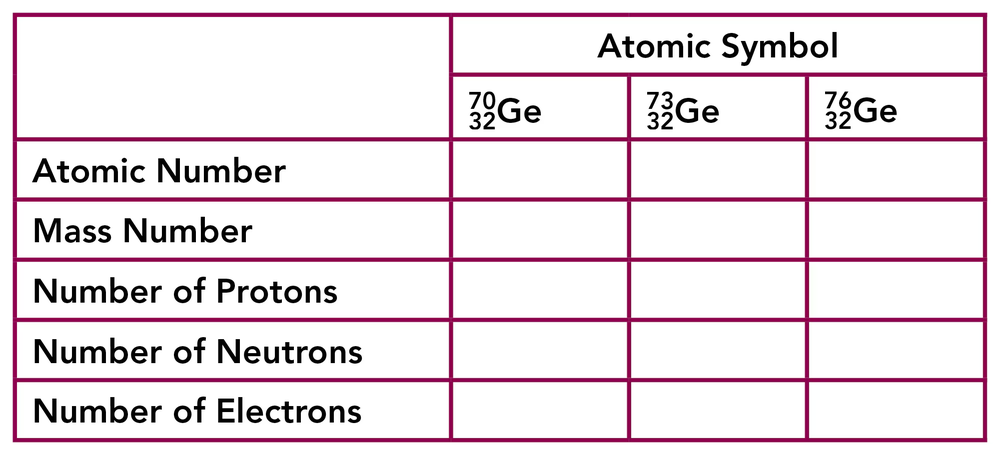
Complete the following table for three of the naturally occurring isotopes of germanium, which is a metalloid used in semiconductor.
Problem 86
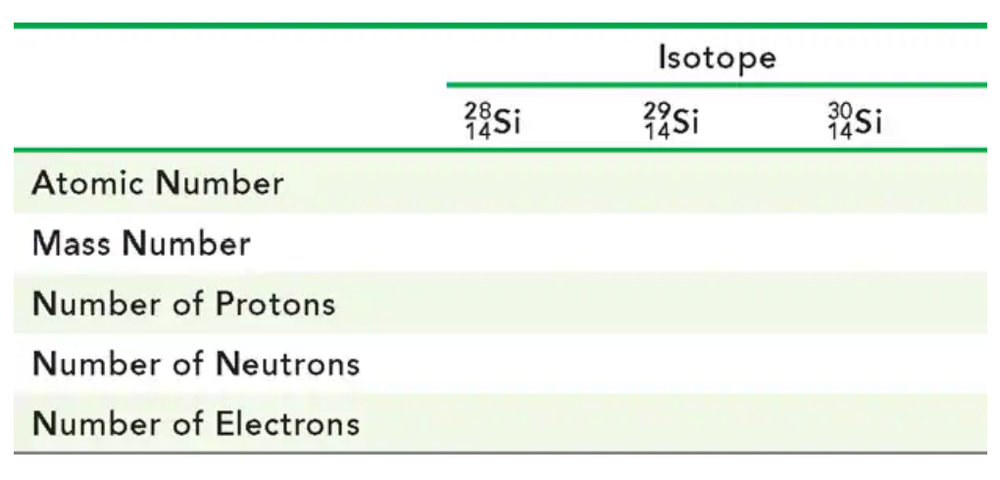
Complete the following table for the three naturally occurring isotopes of silicon, the major component in computer chips:
Problem 87a
For each representation of a nucleus A through E, write the atomic symbol and identify which are isotopes.
a. <IMAGE>
Problem 95a
Complete the following statements:
a. The atomic number gives the number of _____ in the nucleus.
Problem 95b
Complete the following statements:
b. In an atom, the number of electrons is equal to the number of _____.
Problem 95c
Complete the following statements:
c. Sodium and potassium are examples of elements called _____.
Problem 96a
Complete the following statements:
a. The number of protons and neutrons in an atom is also the _____ number.
Problem 96b
Complete the following statements:
b. The elements in Group 7A (17) are called the _____.
Problem 96c
Complete the following statements:
c. Elements that are shiny and conduct heat are called _____.
Problem 97c
Indicate if each of the following statements is true or false:
c. The atomic mass unit is based on a carbon atom with six protons and six neutrons.
Problem 98a
Which of the following molecules will produce the most ATP per mole?
a. glucose or stearic acid (C18)
Problem 98d
Indicate if each of the following statements is true or false:
d. The proton and the electron have about the same mass.
Problem 102h
Write the name and symbol of the element with the following atomic number:
h. 92
Problem 103a,b
Provide the following:
a. the atomic number and symbol of the lightest alkali metal
b. the atomic number and symbol of the heaviest noble gas
Problem 103c,d
Provide the following:
c. the atomic mass and symbol of the alkaline earth metal in Period 3
d. the atomic mass and symbol of the halogen with the fewest electrons
Problem 104a,b
Provide the following:
a. the atomic number and symbol of the lightest alkali metal
b. the atomic number and symbol of the heaviest noble gas
Problem 104c,d
Provide the following:
c. the atomic mass and symbol of the alkaline earth metal in Period 3
d. the atomic mass and symbol of the halogen with the fewest electrons
Problem 113
Why is the ionization energy of Ca higher than K, but lower than that of Mg?
Problem 114
Why is the ionization energy of Cl lower than F, but higher than that of S?
Problem 121e
Give the symbol of the element that has the
e. most metallic character in Group 2A (2)
Problem 122a
Give the symbol of the element that has the
a. largest atomic size in Group 1A (1)
Problem 122b
Give the symbol of the element that has the
b. largest atomic size in Period 4
Problem 122e
Give the symbol of the element that has the
e. least metallic character in Group 4A (14)