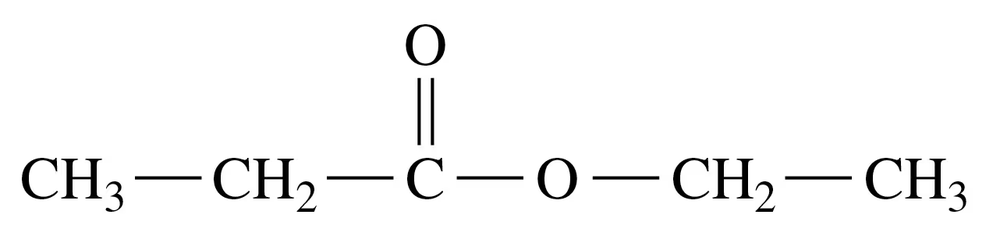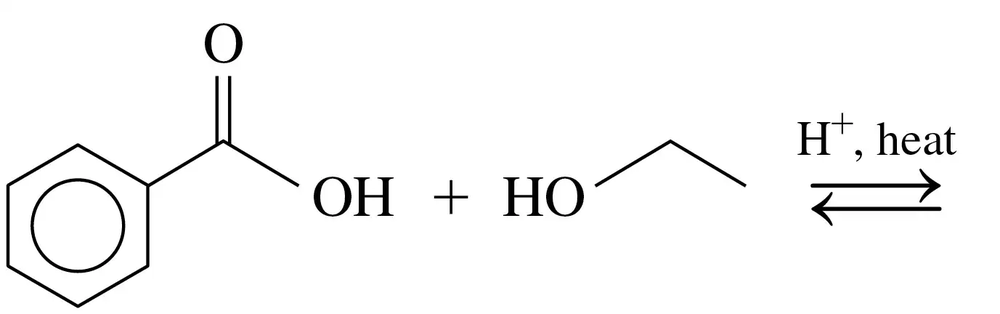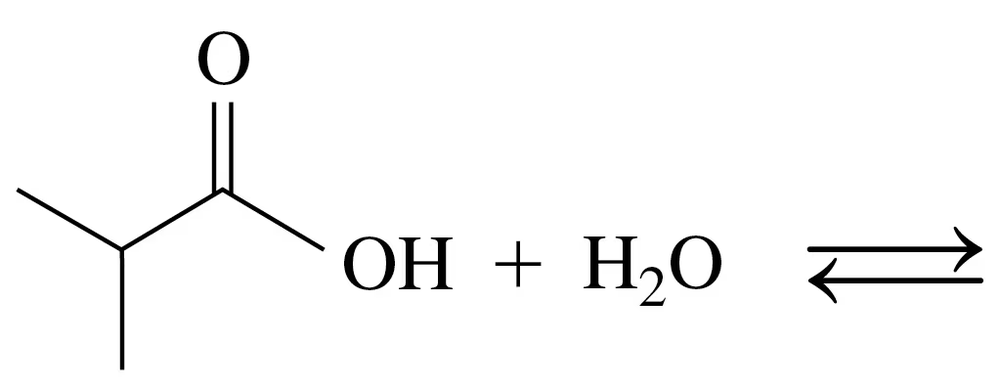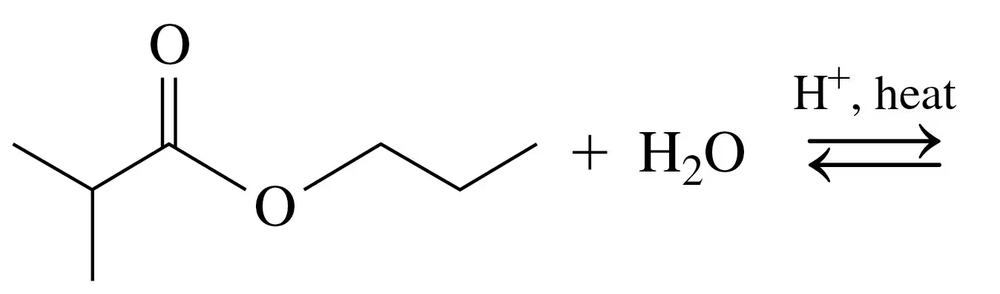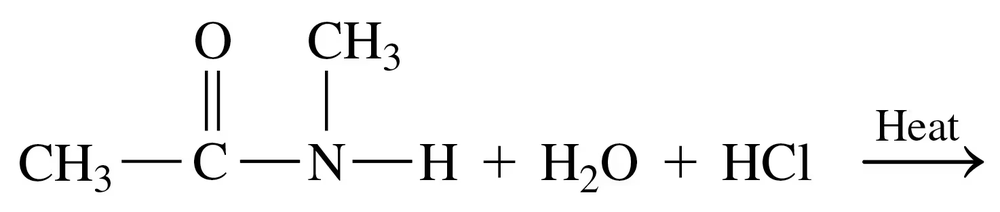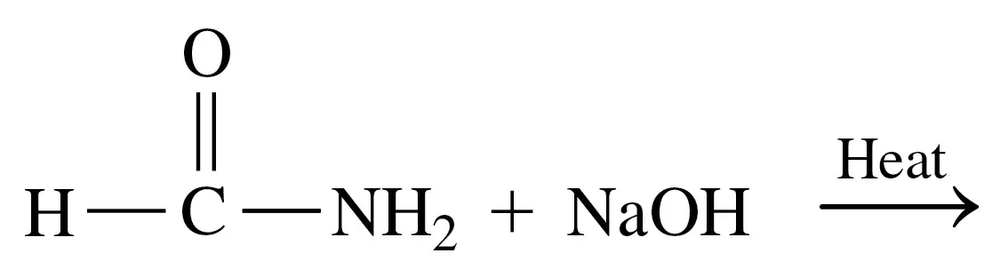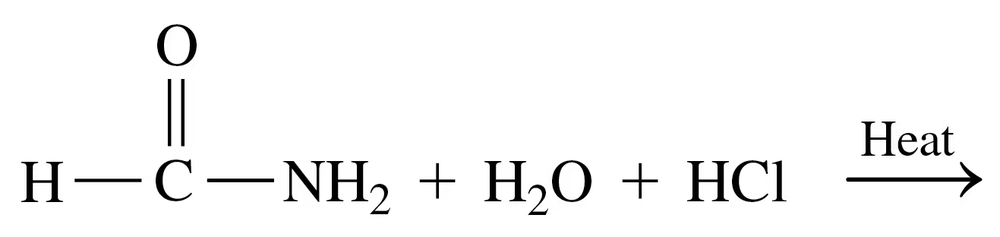Back
BackProblem 57b
Write the IUPAC and common names, if any, for each of the following:
c.
Problem 58b
Write the IUPAC and common names, if any, for each of the following:
c.
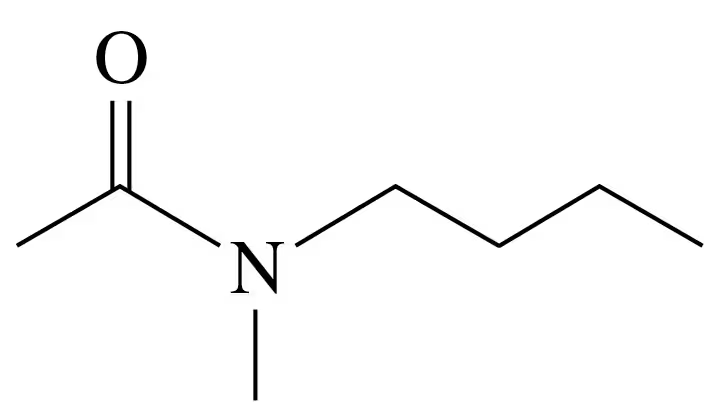
Problem 58e
Write the IUPAC and common names, if any, for each of the following:
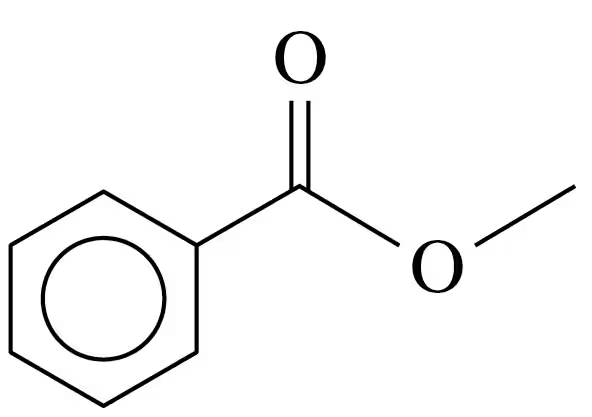
e.
Problem 59c
Draw the condensed structural formulas for a and b and line-angle formulas for c and d:
c. 3-bromopentanoic acid
Problem 60a
Draw the condensed structural formulas for a and b and line-angle formulas for c and d:
a. pentyl formate
Problem 61b
Draw the condensed structural or line-angle formulas for the products of the following:
c.
Problem 62b
Draw the condensed structural or line-angle formulas for the products of the following:
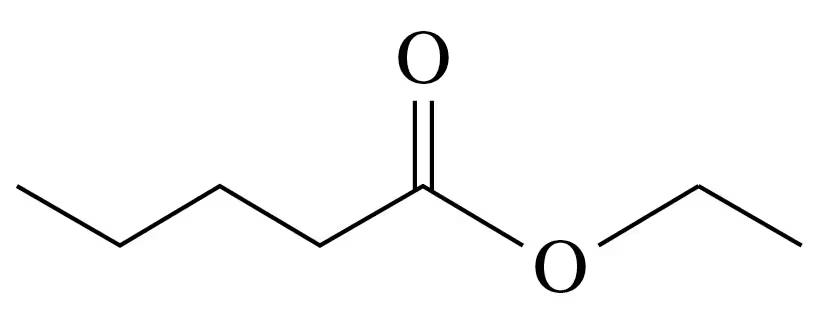
b.
Problem 62d
Draw the condensed structural or line-angle formulas for the products of the following:
d.
Problem 63c
Draw the condensed structural or line-angle formulas for the products of the following:
c.
Problem 64a
Draw the condensed structural or line-angle formulas for the products of the following:
a.
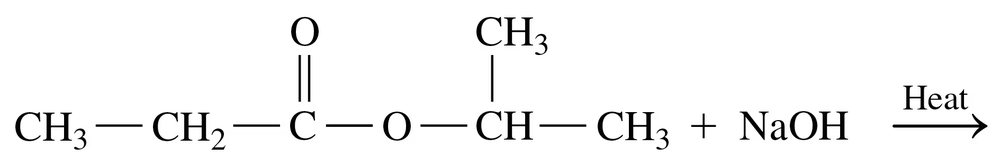
Problem 64b
Draw the condensed structural or line-angle formulas for the products of the following:
b.
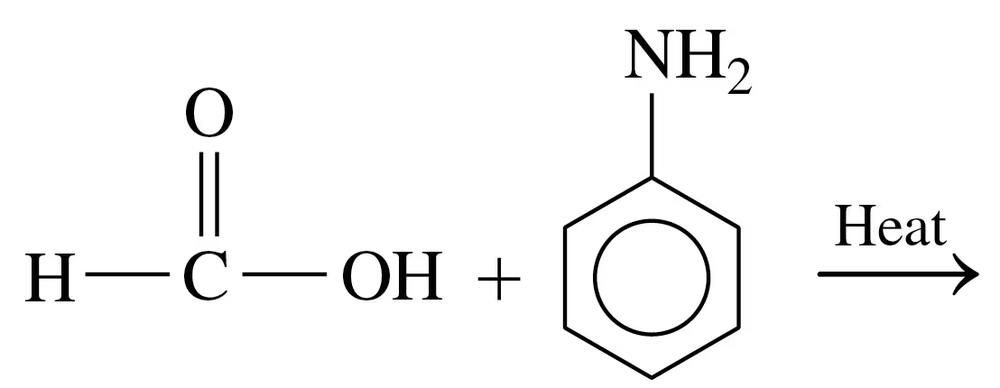
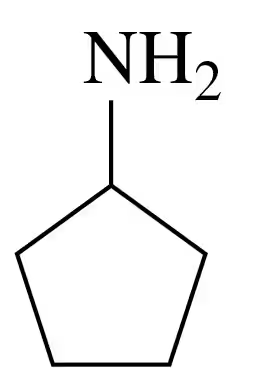
Problem 66c
Write the common name and classify each of the following compounds as primary (1°), secondary (2°), or tertiary (3°):
b.
Problem 67b
Draw the condensed structural or line-angle formula if cyclic, for each of the following:
b. cyclohexylamine
Problem 67d
Draw the condensed structural or line-angle formula if cyclic, for each of the following:
d. N-propylaniline
Problem 68d
Draw the condensed structural or line-angle formula if cyclic, for each of the following:
d. ethylmethylammonium bromide
Problem 69a
Draw the condensed structural or line-angle formulas for the products of the following:
a. CH3–CH2–NH2 + H2O ⇌
Problem 72
Draw the condensed structural formulas for the products of the reaction of aspartate and α-ketoglutarate which is catalyzed by aspartate transaminase (AST).
Problem 72b
Write the IUPAC name for each of the following:
b .
Problem 73a
Draw the condensed structural or line-angle formulas for the products from the hydrolysis of each of the following:
a.
Problem 73b
Draw the condensed structural or line-angle formulas for the products from the hydrolysis of each of the following:
b.
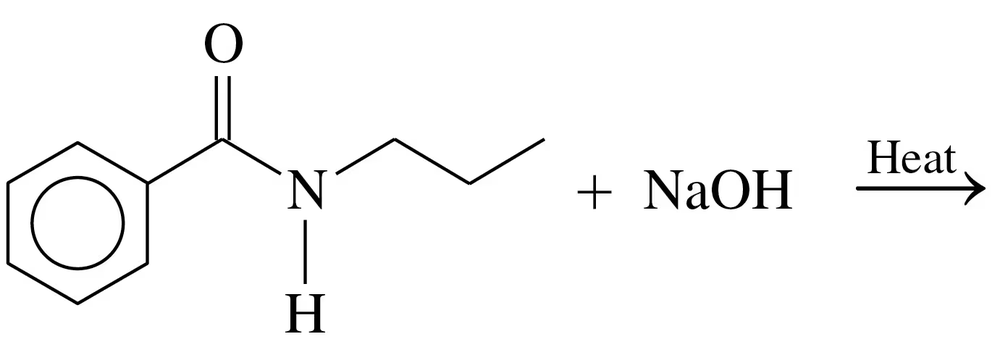
Problem 74c
Draw the condensed structural or line-angle formulas for the products from the hydrolysis of each of the following:
b.
Problem 77a
Draw the line-angle formula and write the IUPAC name for each of the following:
a. A carboxylic acid that has the formula C6H12O2, with no substituents
Problem 79a
Propyl acetate is the ester that gives the odor and smell of pears.
<IMAGE>
a. Draw the condensed structural formula for propyl acetate.
Problem 79b
Propyl acetate is the ester that gives the odor and smell of pears.
b. Use condensed structural formulas to write the balanced chemical equation for the formation of propyl acetate.
Problem 79d
Propyl acetate is the ester that gives the odor and smell of pears.
d. Use condensed structural formulas to write the balanced chemical equation for the base hydrolysis of propyl acetate with NaOH.
Problem 79e
Propyl acetate is the ester that gives the odor and smell of pears.
<IMAGE>
e. How many milliliters of a 0.208 M NaOH solution is needed to completely hydrolyze (saponify) 1.58 g of propyl acetate?
Problem 80c
Ethyl octanoate is a flavor component of mangoes.
c. Use condensed structural formula to write the balanced chemical equation for the acid hydrolysis of ethyl octanoate.
Problem 80e
Ethyl octanoate is a flavor component of mangoes.
<IMAGE>
e. How many milliliters of a 0.315 M NaOH solution is needed to completely hydrolyze (saponify) 2.84 g of ethyl octanoate?
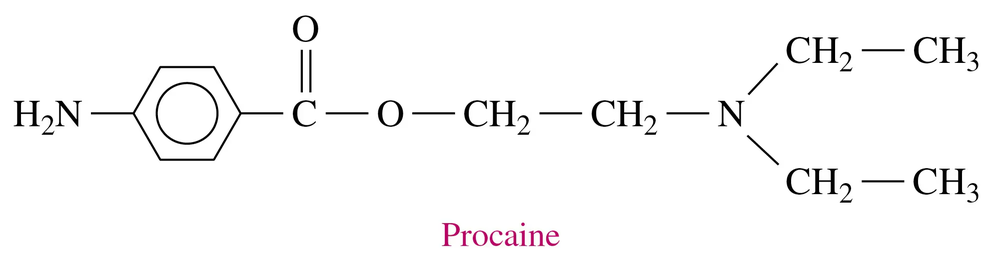
Problem 81a
Novocain, a local anesthetic, is the ammonium salt of procaine.
a. Draw the condensed structural formula for the ammonium salt (procaine hydrochloride) formed when procaine reacts with HCl. (Hint: The tertiary amine reacts with HCl.)
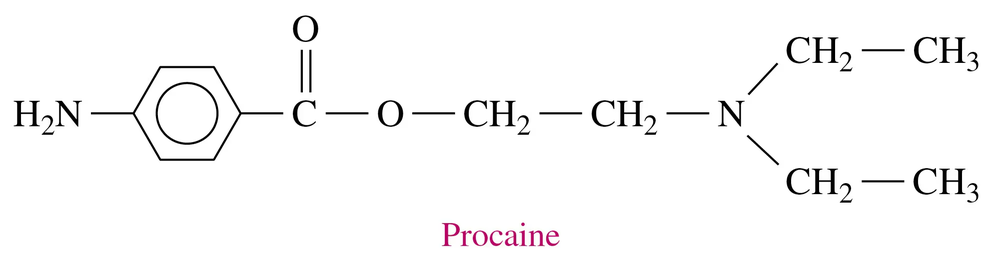
Problem 81b
Novocain, a local anesthetic, is the ammonium salt of procaine.
b. Why is procaine hydrochloride used rather than procaine?