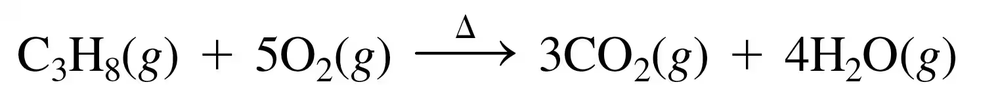Back
BackProblem 1
What is a mole?
Problem 2
What is Avogadro's number?
Problem 11b
Calculate the molar mass for each of the following:
b. C3H6O3
Problem 12c
Calculate the molar mass for each of the following:
c. Fe(ClO4)3
Problem 17a
Calculate the molar mass for each of the following:
a. Al2(SO4)3, antiperspirant
Problem 22d
Calculate the mass, in grams, for each of the following:
d. 0.145 mole of C2H6O
Problem 22e
Calculate the mass, in grams, for each of the following:
e. 2.08 moles of (NH4)2SO4
Problem 35d
Determine whether each of the following chemical equations is balanced or not balanced:
d.
Problem 38c
Balance each of the following chemical equations:
c. Sb2S3(s) + HCl(aq) → SbCl3(aq) + H2S(g)
Problem 39d
Balance each of the following chemical equations:
d. Al(s) + HCl(aq) → H2(g) + AlCl3(aq)
Problem 45c
Identify each of the following as an oxidation or a reduction:
c. Cr3+(aq) + 3e– → Cr(s)