Back
BackProblem 72a
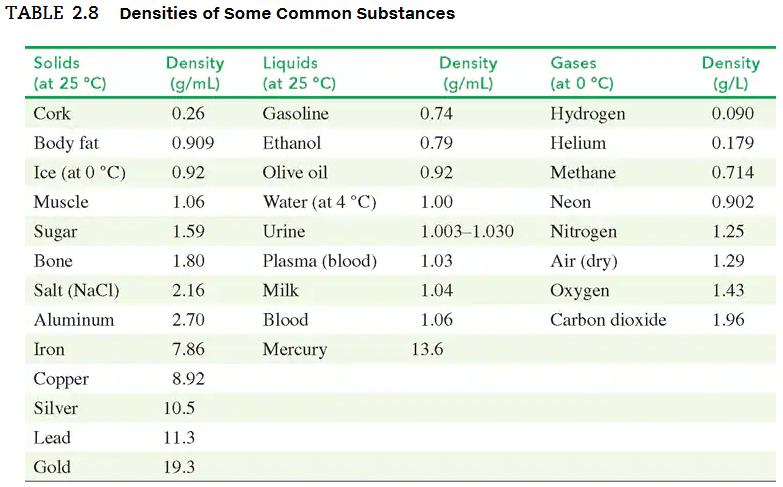
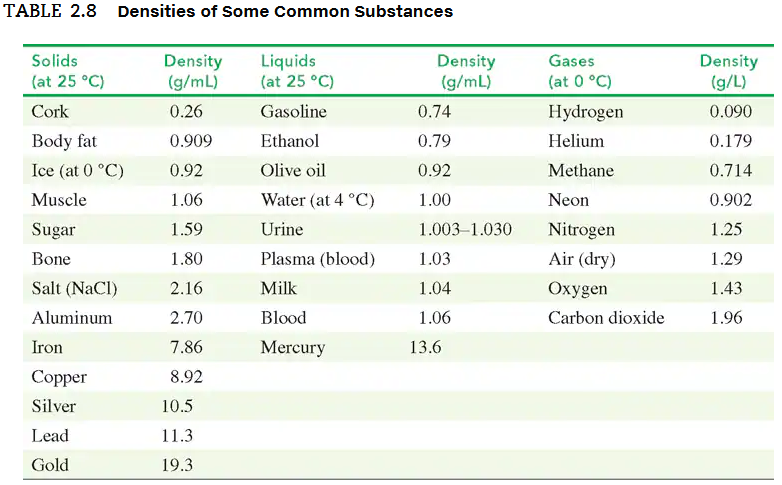
Use the density values in TABLE 2.8 to solve each of the following problems:
a. A graduated cylinder contains 18.0 mL of water. What is the new water level, in milliliters, after 35.6 g of silver metal is submerged in the water?
Problem 75
In an old trunk, you find a piece of metal that you think may be aluminum, silver, or lead. You take it to a lab, where you find it has a mass of 217 g and a volume of 19.2 cm3. Using TABLE 2.8, what is the metal you found?
Problem 77a
Solve each of the following problems:
a. A urine sample has a density of 1.030 g/mL. What is the specific gravity of the sample?
Problem 77c
Solve each of the following problems:
c. The specific gravity of a vegetable oil is 0.92. What is the mass, in grams, of 750 mL of vegetable oil?
Problem 77d
Solve each of the following problems:
d. A bottle containing 325 g of cleaning solution is used to clean hospital equipment. If the cleaning solution has a specific gravity of 0.850, what volume, in milliliters, of solution was used?
Problem 84a
Measure the length of each of the objects in diagrams (a), (b), and (c) using the metric ruler in the figure. Indicate the number of significant figures for each and the estimated digit for each.
a. <IMAGE>
Problem 85
State the temperature on the Celsius thermometer to the correct number of significant figures:
<IMAGE>
Problem 87d
The length of this rug is 38.4 in. and the width is 24.2 in.
<IMAGE>
d. Calculate the area of the rug, in square centimeters, to the correct number of significant figures. (Area = Length x Width)
Problem 88a
A shipping box has a length of 7.00 in., a width of 6.00 in., and a height of 4.00 in.
<IMAGE>
a. What is the length of the box, in centimeters?
Problem 88b
A shipping box has a length of 7.00 in., a width of 6.00 in., and a height of 4.00 in.
<IMAGE>
b. What is the width of the box, in centimeters?
Problem 91
Consider the following solids. The solids A, B, and C represent aluminum (D = 2.70g/mL), and silver (D = 10.5 g/mL). If each has a mass of 10.0 g, what is the identity of each solid?
<IMAGE>
Problem 93
The gray cube has a density of 4.5 g/cm3. Is the density of the green cube the same, lower than, or higher than that of the gray cube?
<IMAGE>
Problem 99
In France, grapes are 1.95 euros per kilogram. What is the cost of grapes, in dollars per pound, if the exchange rate is 1.14 dollars/euro?
Problem 100
In Mexico, avocados are 48 pesos per kilogram. What is the cost, in cents, of an avocado that weighs 0.45 lb if the exchange rate is 18 pesos to the dollar?
Problem 105
The water level in a graduated cylinder initially at 215 mL rises to 285 mL after a piece of lead is submerged. What is the mass, in grams, of the lead?
Problem 106
A graduated cylinder contains 155 mL of water. A 15.0-g piece of iron and a 20.0-g piece of lead are added. What is the new water level, in milliliters, in the cylinder?
Problem 107
How many milliliters of gasoline have a mass of 1.2 kg?
Problem 111
To treat a bacterial infection, a doctor orders 4 tablets of amoxicillin per day for 10 days. If each tablet contains 250 mg of amoxicillin, how many ounces of the medication are given in 10 days?
Problem 120
A 50.0-g silver object and a 50.0-g gold object are both added to 75.5 mL of water contained in a graduated cylinder. What is the new water level, in milliliters, in the cylinder?
Problem 121a
An athlete with a body mass of 65 kg has 3.0% body fat. How many pounds of body fat does that person have?