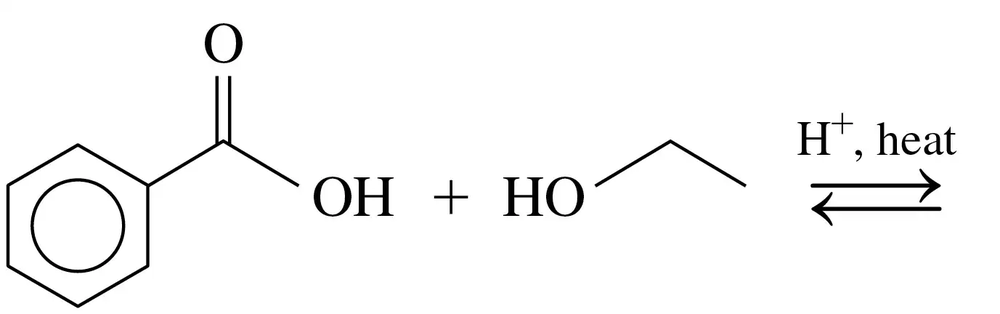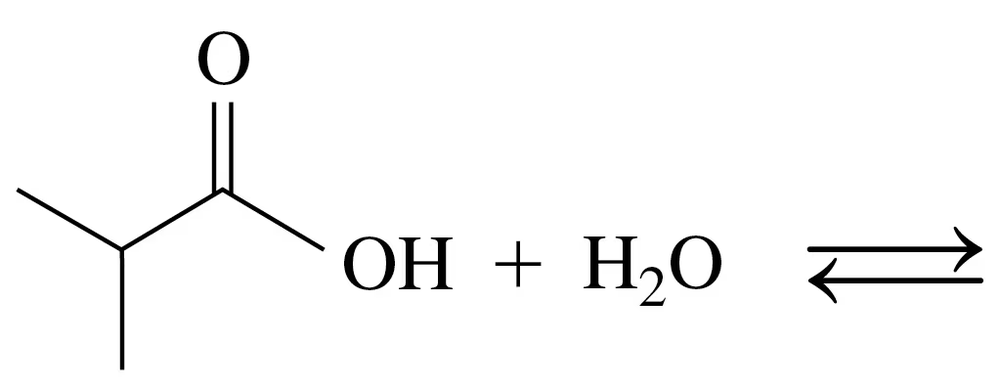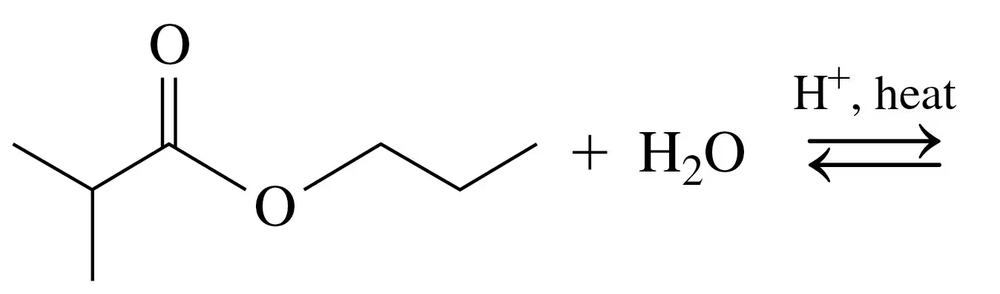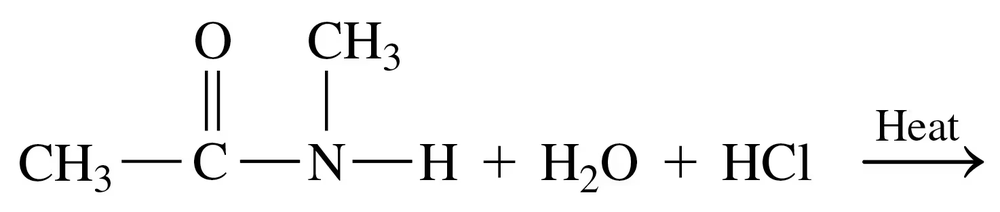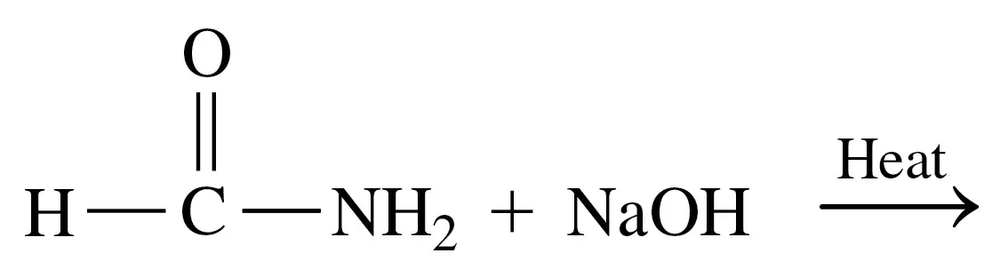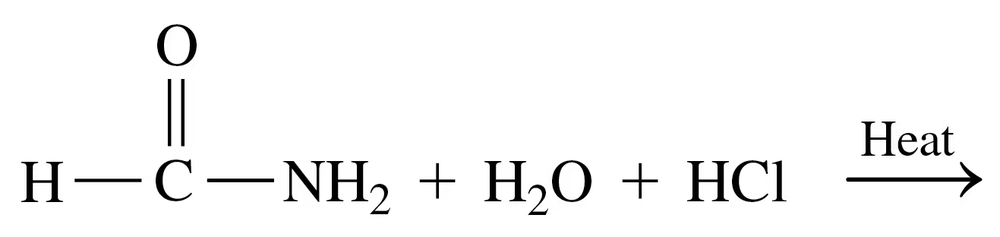Back
BackProblem 58c
Write the IUPAC and common names, if any, for each of the following:
c.
Problem 58e
Write the IUPAC and common names, if any, for each of the following:
e.
Problem 59c
Draw the condensed structural formulas for a and b and line-angle formulas for c and d:
c. 3-bromopentanoic acid
Problem 60a
Draw the condensed structural formulas for a and b and line-angle formulas for c and d:
a. pentyl formate
Problem 61c
Draw the condensed structural or line-angle formulas for the products of the following:
c.
Problem 62b
Draw the condensed structural or line-angle formulas for the products of the following:
b.
Problem 62d
Draw the condensed structural or line-angle formulas for the products of the following:
d.
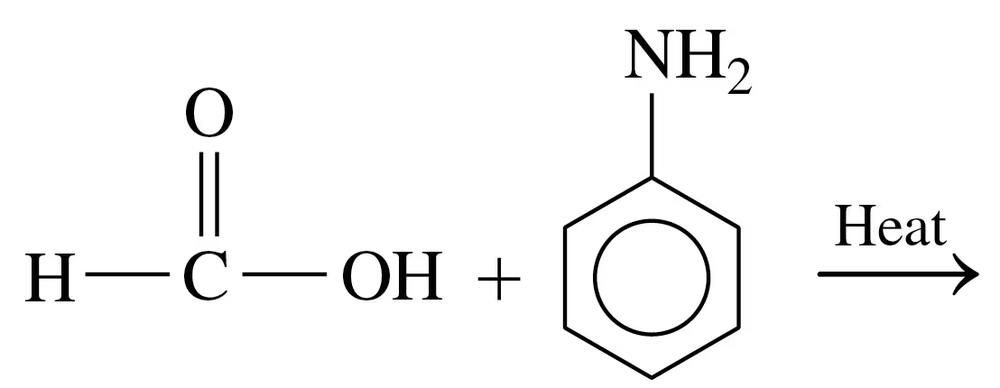
Problem 63c
Draw the condensed structural or line-angle formulas for the products of the following:
c.
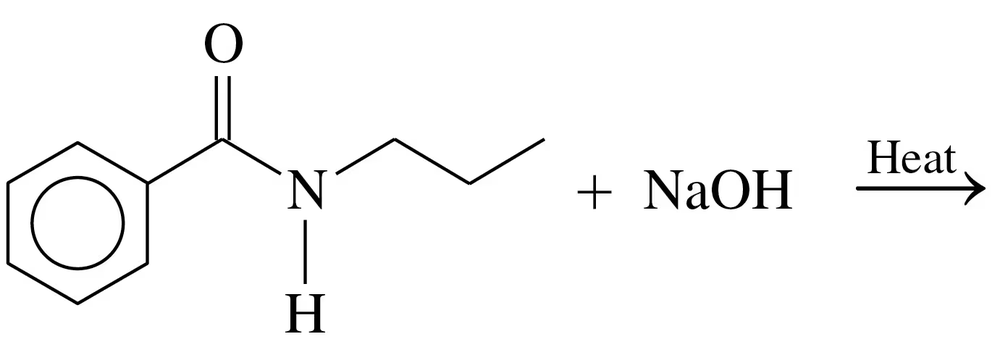
Problem 64a
Draw the condensed structural or line-angle formulas for the products of the following:
a.
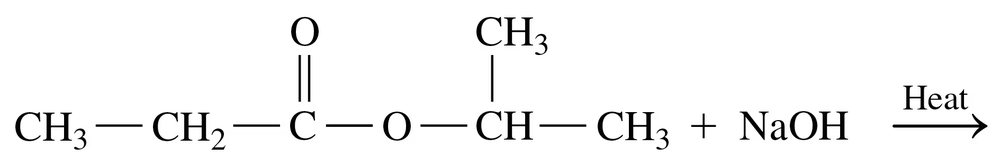
Problem 64b
Draw the condensed structural or line-angle formulas for the products of the following:
b.
Problem 66a
Write the common name and classify each of the following compounds as primary (1°), secondary (2°), or tertiary (3°): (14.5)
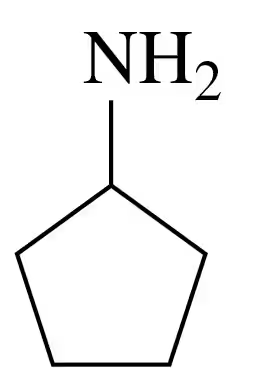
a.
Problem 66b
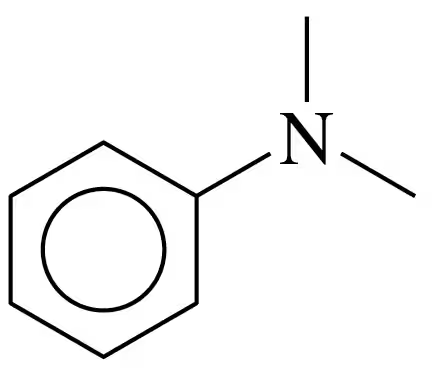
Write the common name and classify each of the following compounds as primary (1°), secondary (2°), or tertiary (3°):
b.
Problem 67b
Draw the condensed structural or line-angle formula if cyclic, for each of the following:
b. cyclohexylamine
Problem 67d
Draw the condensed structural or line-angle formula if cyclic, for each of the following:
d. N-propylaniline
Problem 68d
Draw the condensed structural or line-angle formula if cyclic, for each of the following:
d. ethylmethylammonium bromide
Problem 69a
Draw the condensed structural or line-angle formulas for the products of the following:
a. CH3–CH2–NH2 + H2O ⇌
Problem 72b
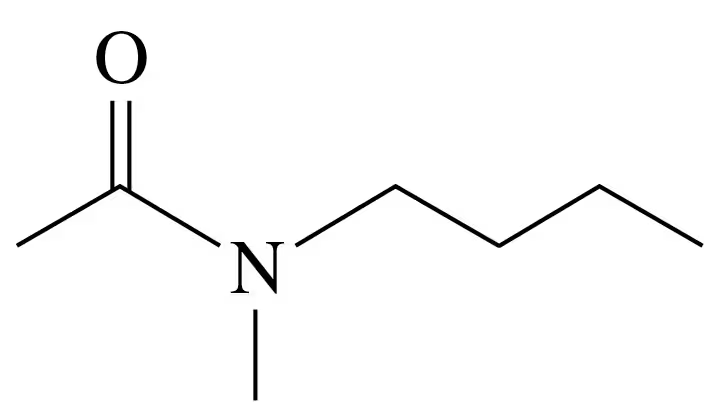
Write the IUPAC name for each of the following:
b .
Problem 73a
Draw the condensed structural or line-angle formulas for the products from the hydrolysis of each of the following:
a.
Problem 73b
Draw the condensed structural or line-angle formulas for the products from the hydrolysis of each of the following:
b.
Problem 74b
Draw the condensed structural or line-angle formulas for the products from the hydrolysis of each of the following:
b.
Problem 77a
Draw the line-angle formula and write the IUPAC name for each of the following:
a. A carboxylic acid that has the formula C6H12O2, with no substituents
Problem 79a
Propyl acetate is the ester that gives the odor and smell of pears.
<IMAGE>
a. Draw the condensed structural formula for propyl acetate.
Problem 79b
Propyl acetate is the ester that gives the odor and smell of pears.
<IMAGE>
b. Write the balanced chemical equation for the formation of propyl acetate.
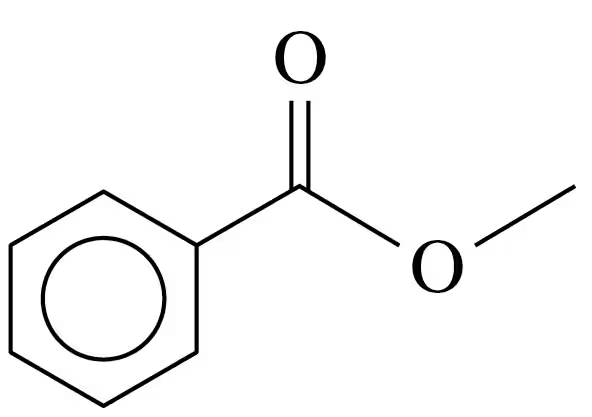
Problem 79d
Propyl acetate is the ester that gives the odor and smell of pears.
<IMAGE>
d. Write the balanced chemical equation for the base hydrolysis of propyl acetate with NaOH.
Problem 79e
Propyl acetate is the ester that gives the odor and smell of pears.
<IMAGE>
e. How many milliliters of a 0.208 M NaOH solution is needed to completely hydrolyze (saponify) 1.58 g of propyl acetate?
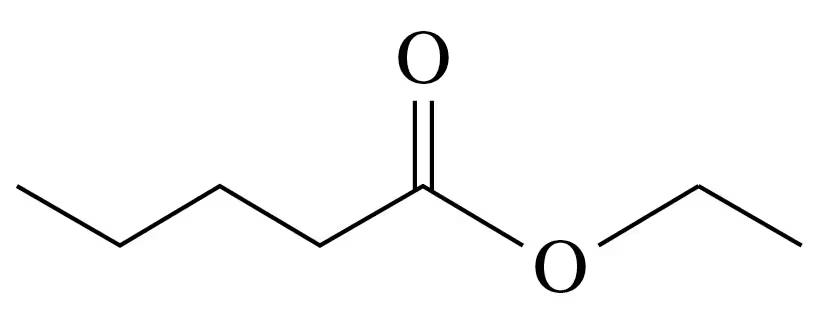
Problem 80c
Ethyl octanoate is a flavor component of mangoes.
<IMAGE>
c. Write the balanced chemical equation for the acid hydrolysis of ethyl octanoate.
Problem 80e
Ethyl octanoate is a flavor component of mangoes.
<IMAGE>
e. How many milliliters of a 0.315 M NaOH solution is needed to completely hydrolyze (saponify) 2.84 g of ethyl octanoate?
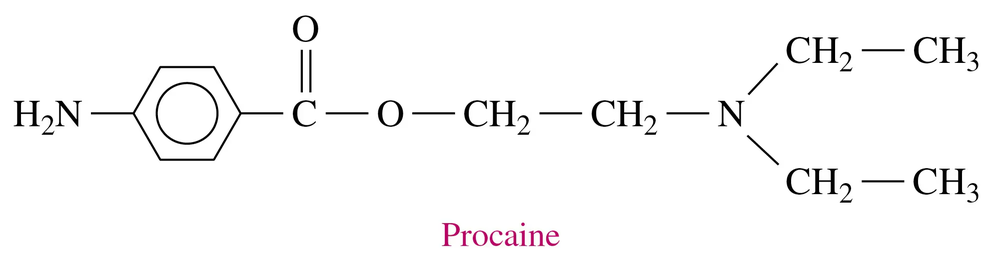
Problem 81a
Novocain, a local anesthetic, is the ammonium salt of procaine.
a. Draw the condensed structural formula for the ammonium salt (procaine hydrochloride) formed when procaine reacts with HCl. (Hint: The tertiary amine reacts with HCl.)
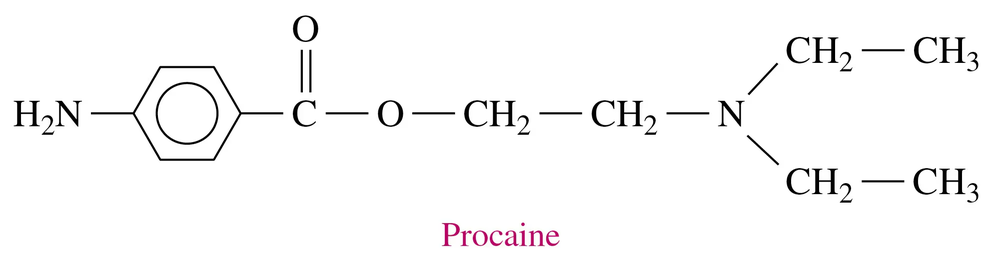
Problem 81b
Novocain, a local anesthetic, is the ammonium salt of procaine.
b. Why is procaine hydrochloride used rather than procaine?
Problem 82a
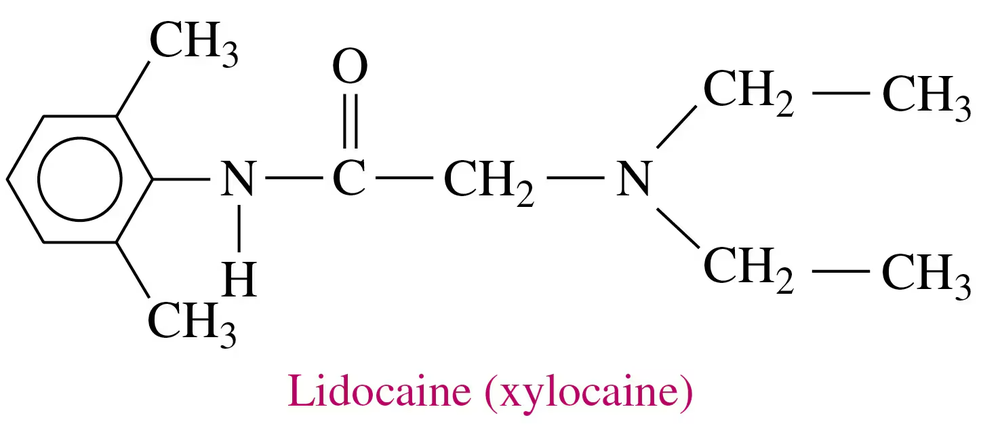
Lidocaine (xylocaine) is used as a local anesthetic and cardiac depressant.
a. Draw the condensed structural formula for the ammonium salt formed when lidocaine reacts with HCl.