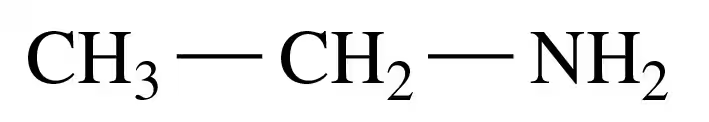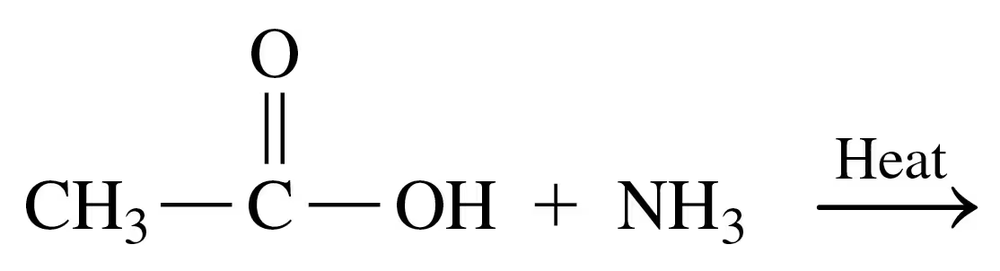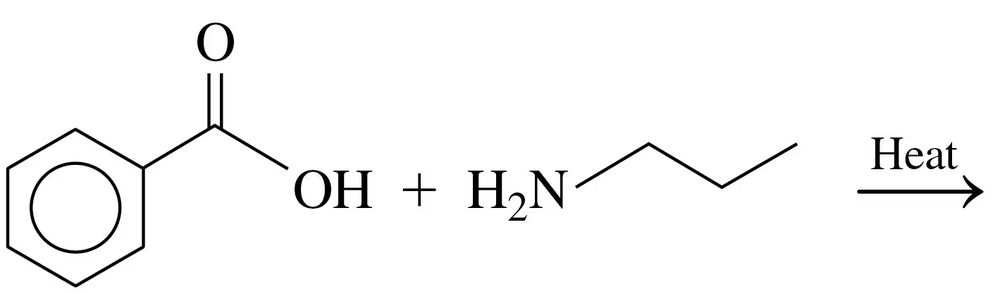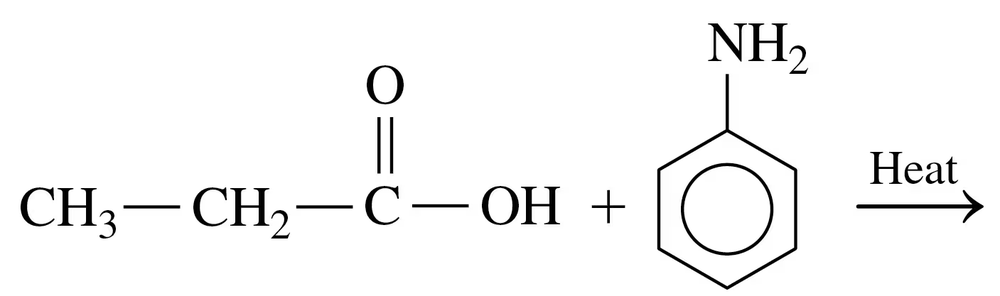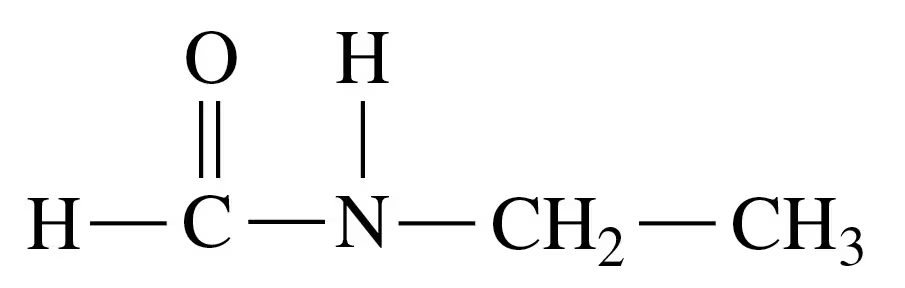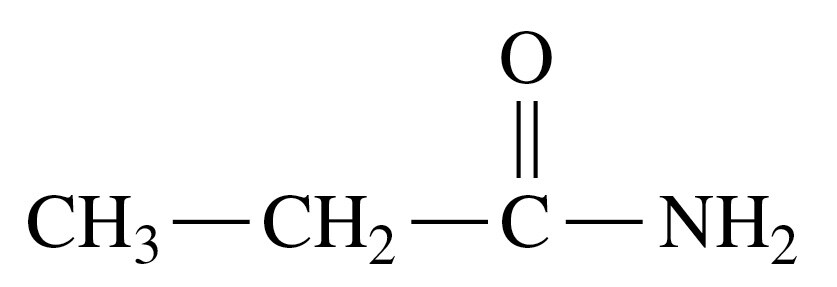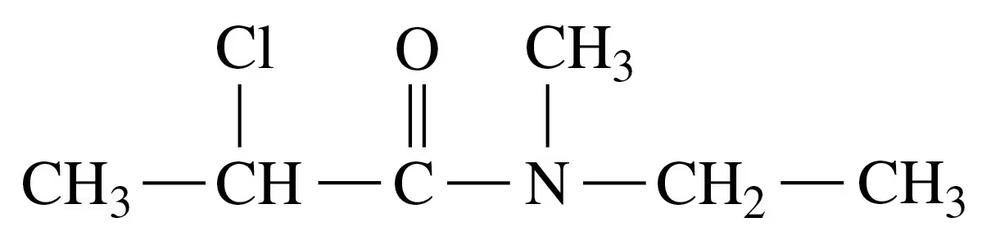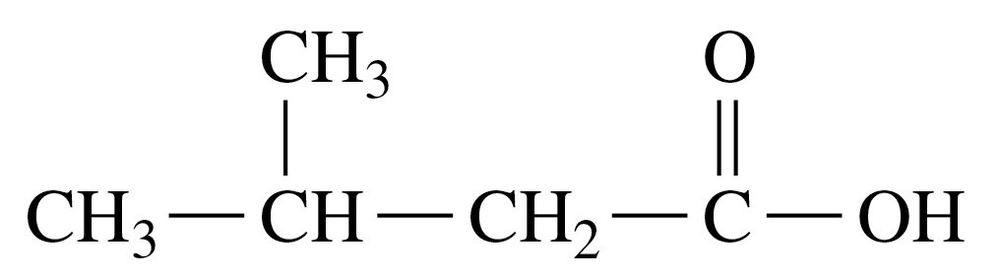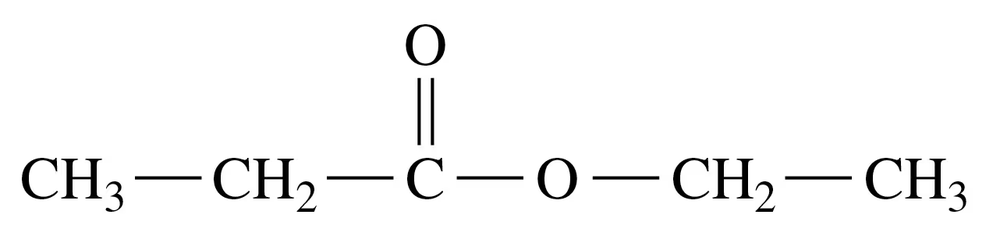Back
BackProblem 33c
Classify each of the following amines as primary (1°), secondary (2°), or tertiary (3°):
c
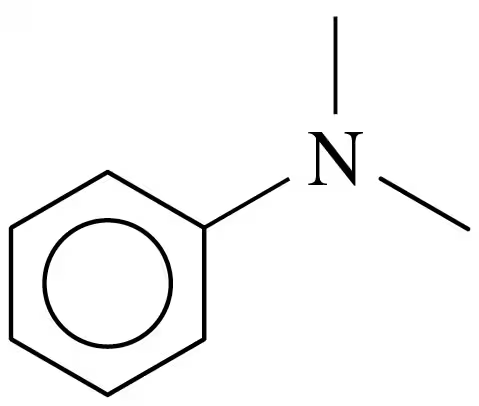
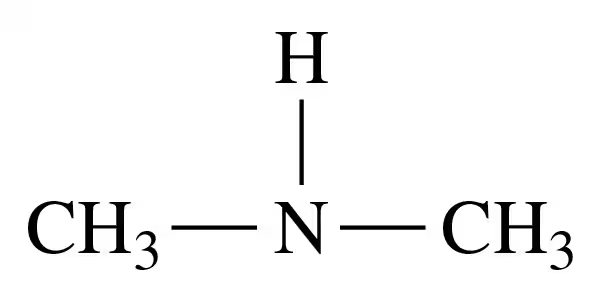
Problem 33d
Classify each of the following amines as primary (1°), secondary (2°), or tertiary (3°):
d.
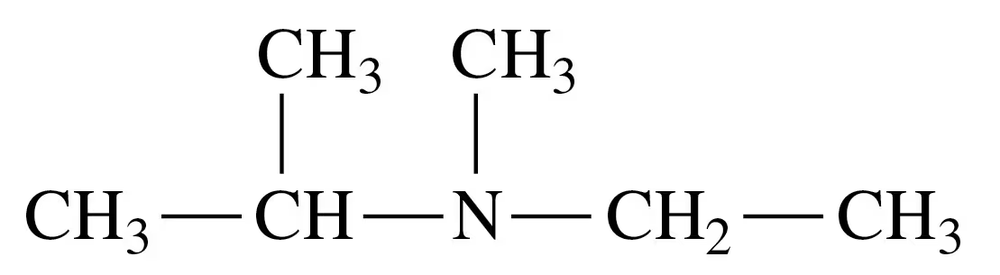
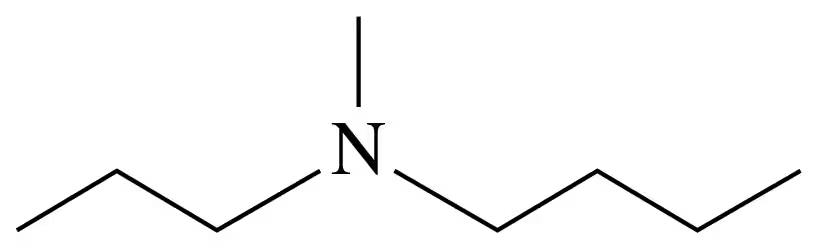
Problem 34c
Classify each of the following amines as primary (1°), secondary (2°), or tertiary (3°):
c.
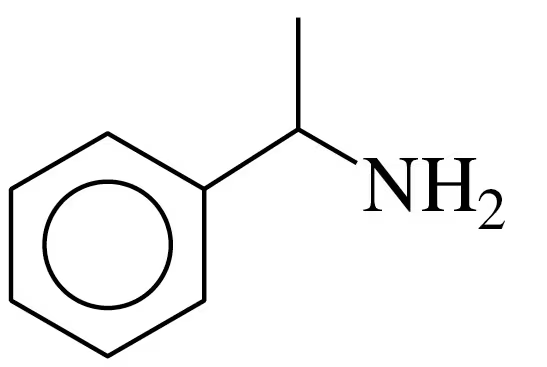
Problem 35a
Indicate if each of the following is soluble in water. Explain.
a.
Problem 35b
Indicate if each of the following is soluble in water. Explain.
b.
Problem 35c
Indicate if each of the following is soluble in water. Explain.
c.
Problem 36d
Indicate if each of the following is soluble in water. Explain.
d.
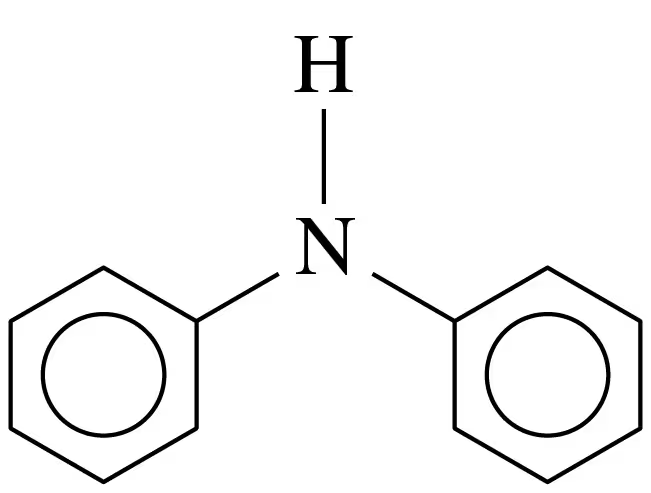
Problem 37c
Write the balanced chemical equations for the (1) reaction of each of the following amines with water and (2) neutralization with HCl:
c. aniline
Problem 38b
Write the balanced chemical equations for the (1) reaction of each of the following amines with water and (2) neutralization with HBr:
b. propylamine
Problem 38c
Write the balanced chemical equations for the (1) reaction of each of the following amines with water and (2) neutralization with HBr:
c. N-methylaniline
Problem 39a
Draw the condensed structural or line-angle formula for the amide formed in each of the following reactions:
a.
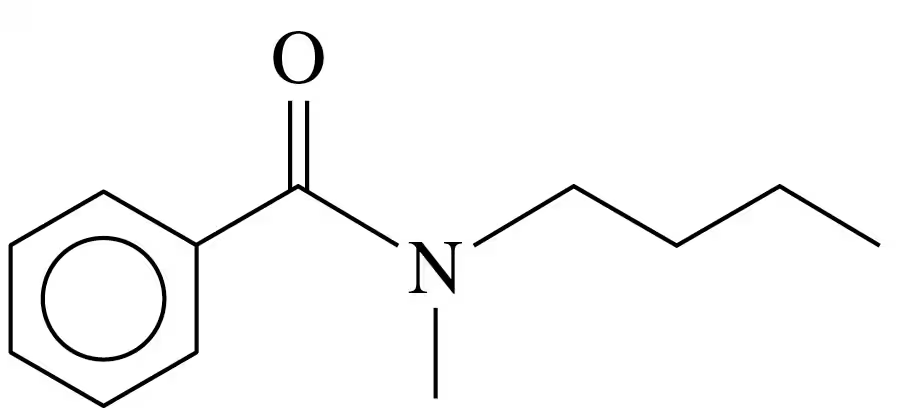
Problem 39c
Draw the condensed structural or line-angle formula for the amide formed in each of the following reactions:
c.
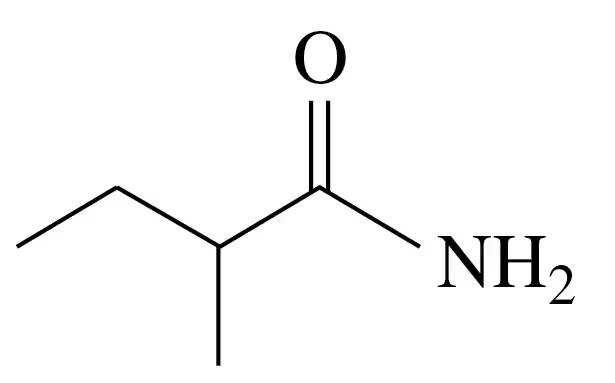
Problem 40c
Draw the condensed structural or line-angle formula for the amide formed in each of the following reactions:
c.
Problem 41c
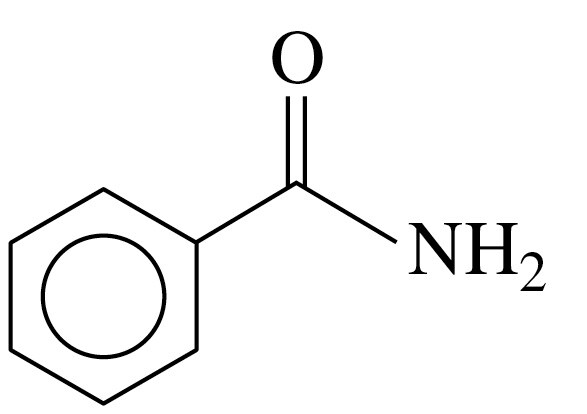
Write the IUPAC and common names, if any, for each of the following amides:
c.
Problem 43b
Draw the condensed structural formula for each of the following amides:
b. 2-methylpentanamide
Problem 44a
Draw the condensed structural formula for each of the following amides:
a. heptanamide
Problem 44c
Draw the condensed structural formula for each of the following amides:
c. 3-methylbutyramide
Problem 45b
Draw the condensed structural or line-angle formulas for the products from the hydrolysis of each of the following amides with HCl:
b.
Problem 45d
Draw the condensed structural or line-angle formulas for the products from the hydrolysis of each of the following amides with HCl:
d.
Problem 46a
Draw the condensed structural or line-angle formulas for the products from the hydrolysis of each of the following amides with NaOH:
a.
Problem 46c
Draw the condensed structural or line-angle formulas for the products from the hydrolysis of each of the following amides with NaOH:
c.
Problem 46d
Draw the condensed structural or line-angle formulas for the products from the hydrolysis of each of the following amides with NaOH:
d.
Problem 49
Draw the condensed structural formulas and write the IUPAC names for two structural isomers of the carboxylic acids that have the molecular formula C4H8O2.
Problem 51a
The ester methyl butanoate has the odor and flavor of strawberries.
a. Draw the condensed structural formula for methyl butanoate.
<IMAGE>
Problem 51c
The ester methyl butanoate has the odor and flavor of strawberries.
c. Write the balanced chemical equation for the acid hydrolysis of methyl butanoate.
<IMAGE>
Problem 52b
Methyl benzoate, which smells like pineapple guava, is used to train detection dogs.
b. Write the IUPAC name for the carboxylic acid and alcohol used to prepare methyl benzoate.
<IMAGE>
Problem 55
There are four amine isomers with the molecular formula C3H9N. Draw their condensed structural formulas, write the common name, and classify each as a primary (1°), secondary (2°), or tertiary (3°) amine.
Problem 56
There are four amide isomers with the molecular formula C3H7NO. Draw their condensed structural formulas and write the IUPAC name for each.
Problem 57a
Write the IUPAC and common names, if any, for each of the following:
a.
Problem 57c
Write the IUPAC and common names, if any, for each of the following:
c.