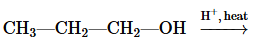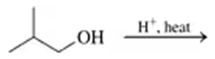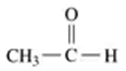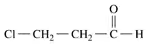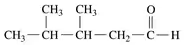Back
BackProblem 50b
Which compound in each pair would be more soluble in water? Explain.
b. 2-propanol or 2-pentanol
Problem 50c
Which compound in each pair would be more soluble in water? Explain.
c. methyl propyl ether or 1-butanol
Problem 51a
Draw the condensed structural or line-angle formula for the alkene, aldehyde, or ketone product of each of the following reactions:
a.
Problem 52a
Draw the condensed structural or line-angle formula for the alkene, aldehyde, or ketone product of each of the following reactions:
a.
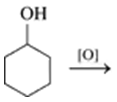
Problem 52c
Draw the condensed structural or line-angle formula for the alkene, aldehyde, or ketone product of each of the following reactions:
c.
Problem 53b
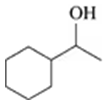
Draw the condensed structural or line-angle formula for the alcohol produced when hydrogen and a nickel catalyst reduce each of the following:
b.
Problem 54a
Draw the condensed structural or line-angle formula for the alcohol produced when hydrogen and a nickel catalyst reduce each of the following:
a.
Problem 54b
Draw the condensed structural or line-angle formula for the alcohol produced when hydrogen and a nickel catalyst reduce each of the following:
b.
Problem 55a
Give the IUPAC name for each of the following:
a.
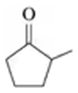
Problem 55b
Give the IUPAC name for each of the following:
b.
Problem 55c
Give the IUPAC name for each of the following:
c.
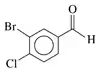
Problem 57a
Draw the condensed structural or line-angle formula, if cyclic, for each of the following:
a. 4-chlorobenzaldehyde
Problem 57b
Draw the condensed structural or line-angle formula, if cyclic, for each of the following:
b. 3-chloropropionaldehyde
Problem 57d
Draw the condensed structural or line-angle formula, if cyclic, for each of the following:
d. 3-methylhexanal
Problem 58d
Draw the condensed structural or line-angle formula, if cyclic, for each of the following:
d. 3,5-dimethylhexanal
Problem 62d
Draw the condensed structural or line-angle formula for the ketone or carboxylic acid product when each of the following is oxidized:
d.
Problem 63
Draw the condensed structural formulas and give the IUPAC names for all the alcohols that have the formula C5H12O.
Problem 64
Draw the condensed structural formulas and give the IUPAC names for all the aldehydes and ketones that have the formula C5H10O. (12.3)
Problem 65
A compound with the formula C4H8O is synthesized from 2-methyl-1-propanol and oxidizes easily to give a carboxylic acid. Draw the condensed structural formula and give the IUPAC name for the compound.
Problem 66
A compound with the formula C₅H₁₀O oxidizes to give 3-pentanone. Draw the condensed structural formula and give the IUPAC name for the compound. (12.3, 12.4)
Problem 66a
A compound with the formula C5H10O oxidizes to give 3-pentanone. Draw the condensed structural formula and give the IUPAC name for the compound.
Problem 67
Compound A is a primary alcohol whose formula is C3H8O. When compound A is heated with strong acid, it dehydrates to form compound B (C3H6). When compound A is oxidized, compound C (C3H6O) forms. Draw the condensed structural formulas and give the IUPAC names for compounds A, B, and C.
Problem 68
Compound X is a secondary alcohol whose formula is C3H8O. When compound X is heated with strong acid, it dehydrates to form compound Y (C3H6). When compound X is oxidized, compound Z (C3H6O) forms, which cannot be oxidized further. Draw the condensed structural formulas and give the IUPAC names for compounds X, Y, and Z.