Back
BackProblem 2a
Indicate whether each of the following statements is characteristic of an acid, a base, or both:
a. neutralizes acids
Problem 6f
Write formulas for each of the following acids and bases:
f. hypochlorous acid
Problem 7a
Identify the reactant that is a Brønsted–Lowry acid and the reactant that is a Brønsted–Lowry base in each of the following:
a. HI(aq) + H2O(l) → I-(aq) + H3O+(aq)
Problem 9b
Write the formula for the conjugate base for each of the following acids:
b. H2O
Problem 10a
Write the formula for the conjugate base for each of the following acids:
a. HCO3-
Problem 10c
Write the formula for the conjugate base for each of the following acids:
c. HPO42-
Problem 11a
Write the formula for the conjugate acid for each of the following bases:
a. CO32-
Problem 11c
Write the formula for the conjugate acid for each of the following bases:
c. H2PO4-
Problem 13b
Identify the Brønsted–Lowry acid–base pairs in each of the following equations:
b. NH4+(aq) + H2O(l) ⇄ NH3(aq) + H3O+(aq)
Problem 15a
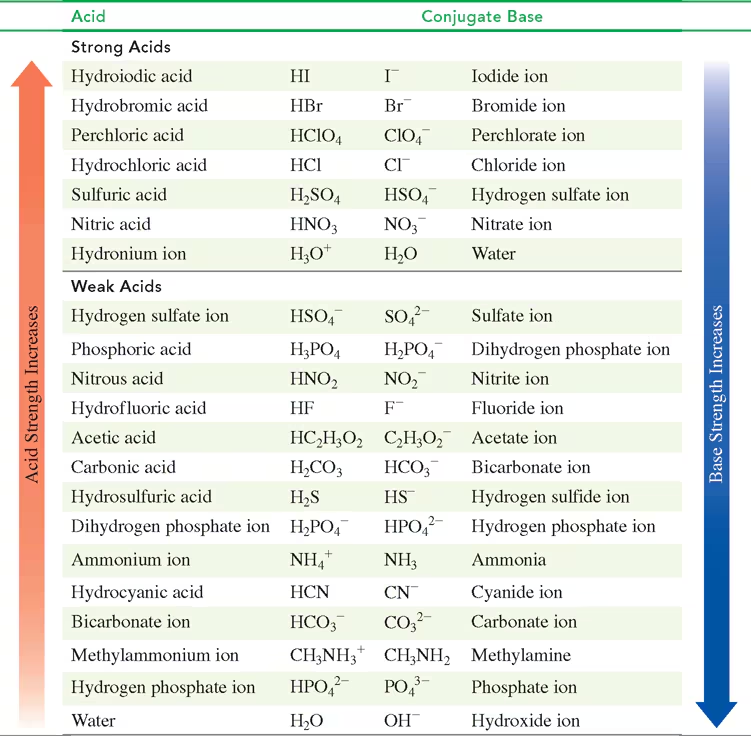
Using TABLE 10.3, identify the stronger acid in each of the following pairs:
a. HBr or HNO2
Problem 15b
Using TABLE 10.3, identify the stronger acid in each of the following pairs:
b. H3PO4 or HSO4-
Problem 19
What is meant by the term reversible reaction?
Problem 27
In an acidic solution, how does the concentration of H3O+ compare to the concentration of OH-?
Problem 28
If a base is added to pure water, why does the [H3O+] decrease?
Problem 33d
Calculate the [H3O+] of each aqueous solution with the following [OH-]:
d. bile, 2.5 × 10-6 M
Problem 34a
Calculate the [H3O+] of each aqueous solution with the following [OH-]:
a. baking soda, 1.0 × 10-6 M
Problem 35c
State whether each of the following is acidic, basic, or neutral:
c. drain cleaner, pH 11.2
Problem 37
Why does a neutral solution have a pH of 7.0?
Problem 38
If you know the [OH-], how can you determine the pH of a solution?
Problem 39c
Calculate the pH of each solution given the following:
c. [OH-] = 1 × 10-5 M
Problem 39f
Calculate the pH of each solution given the following:
f. [OH-] = 8.2 × 10-4 M
Problem 41
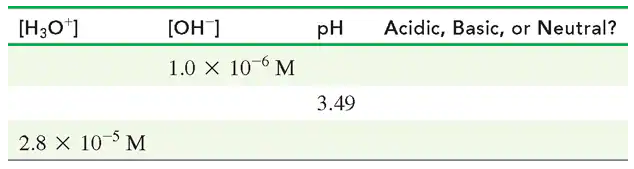
Complete the following table:
Problem 42
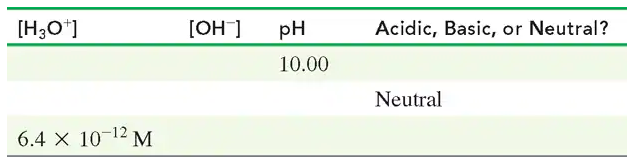
Complete the following table:
Problem 45a
Complete and balance the equation for each of the following reactions:
a. ZnCO3(s) + HBr(aq) →
Problem 45b
Complete and balance the equation for each of the following reactions:
b. Zn(s) + HCl(aq) →
Problem 45c
Complete and balance the equation for each of the following reactions:
c. HCl(aq) + NaHCO3(s) →
Problem 45d
Complete and balance the equation for each of the following reactions:
d. H2SO4(aq) + Mg(OH)2(s) →
Problem 46b
Complete and balance the equation for each of the following reactions:
b. Ca(s) + H2SO4(aq) →
Problem 46c
Complete and balance the equation for each of the following reactions:
c. H2SO4(aq) + Ca(OH)2(s) →
Problem 47a
Balance each of the following neutralization equations:
a. HCl(aq) + Mg(OH)2(s) → H2O(l) + MgCl2(aq)