Textbook Question
Explain the difference between an aldose and a ketose.

 Verified step by step guidance
Verified step by step guidance

Explain the difference between an aldose and a ketose.
Describe the properties of soluble fiber.
Name the functional groups present in aldoses.
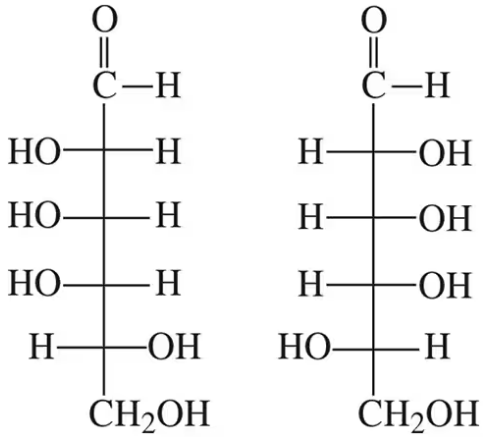
How are the following pairs of carbohydrates, shown in a Fischer projection, related to each other? Are they structural isomers, enantiomers, diastereomers, or epimers? Identify each as the D- or L-isomer.
(b)
How are the following pairs of carbohydrates, shown in a Fischer projection, related to each other? Are they structural isomers, enantiomers, diastereomers, or epimers?
(a)
Draw the Fischer projection of the C3 epimer of D-glucose. Compare your structure with those in Table 6.1 and give the name of this compound.