Back
BackProblem 1
Describe the difference between a Lewis structure and a condensed structure in terms of atoms and bonds shown in the structures.
Problem 3
Explain why it is not possible to draw a skeletal structure for methane.
Problem 5b
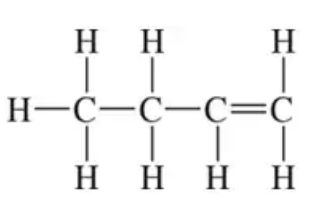
Draw a skeletal structure for each of the following compounds:
(b)
Problem 6b
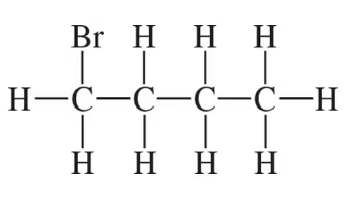
Draw a skeletal structure for each of the following compounds:
(b)
Problem 8b
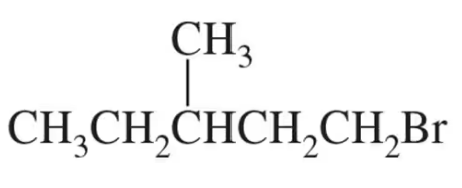
Draw a Lewis structure for each of the following compounds:
(b)
Problem 9b
Draw a condensed structure for each of the following compounds:
(b)
Problem 11b
Name the straight-chain alkanes or cycloalkanes whose structure or formula is shown:
(b) C6H12
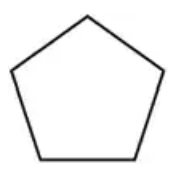
Problem 12b
Name the straight-chain alkanes or cycloalkanes whose structure or formula is shown:
(b)
Problem 13b
Write the condensed structure for the straight-chain alkanes shown:
(b) methane
Problem 14c
Write the condensed structure for the straight-chain alkanes shown:
(c) hexane
Problem 15c
Write the skeletal structure for the alkane or cycloalkane shown:
(c) CH3CH2CH2CH2CH2CH2CH2CH3
Problem 17a
Identify the family of hydrocarbon present in the following:
(a) H3CC≡CH
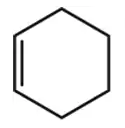
Problem 17c
Identify the family of hydrocarbon present in the following:
(c)
Problem 18a
Identify the family of hydrocarbon present in the following:
(a) CH3CH2CH=CH2
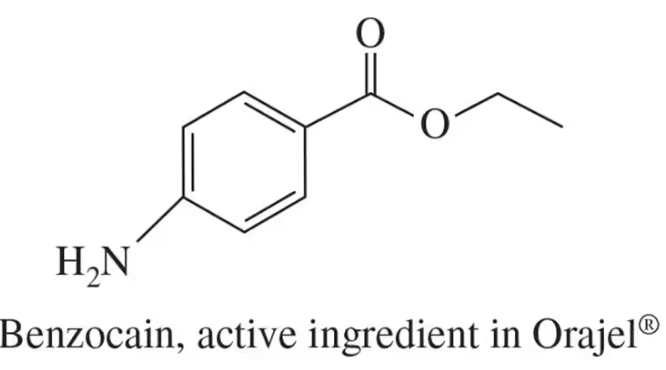
Problem 19a
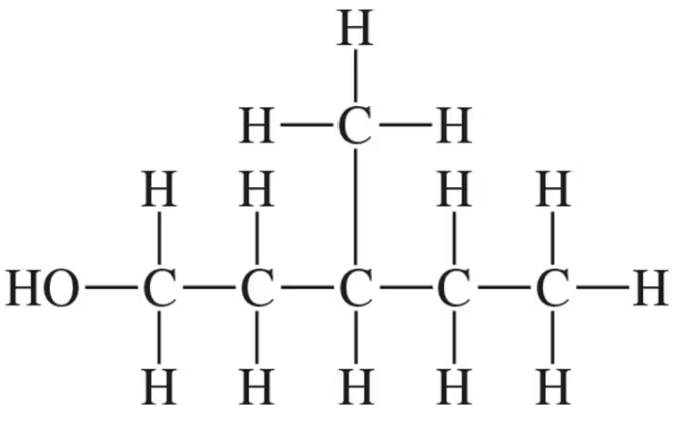
Identify all the functional groups present in the following:
(a)