Textbook Question
Identify the family of hydrocarbon present in the following:
(c)

 Verified step by step guidance
Verified step by step guidance
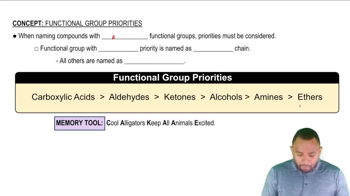
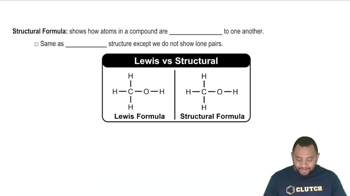

Identify the family of hydrocarbon present in the following:
(c)
Identify the family of hydrocarbon present in the following:
(a) CH3CH2CH=CH2
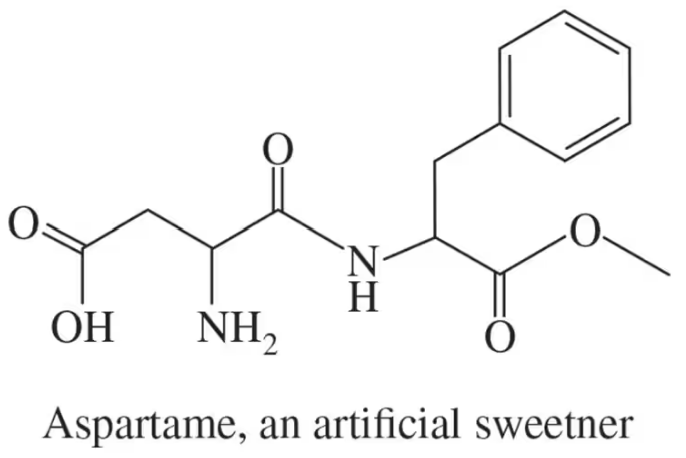
Identify all the functional groups present in the following:
(a)
The most prevalent fatty acid in coconut oil is lauric acid, a saturated fatty acid containing 12 carbons. Draw lauric acid in skeletal structure.
Draw the condensed structural formula for each of the following alkyl groups:
(b) methyl
Give the correct name for each of the following substituents:
(c) I―