Back
BackProblem 17a
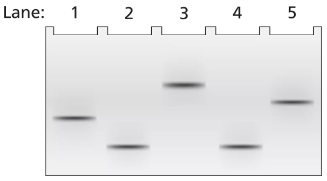
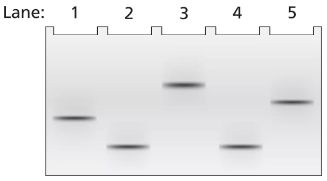
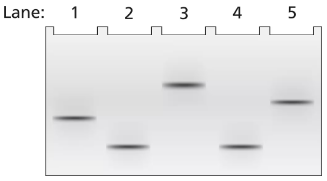
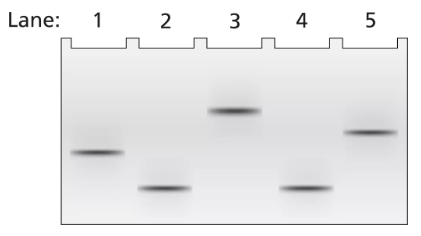
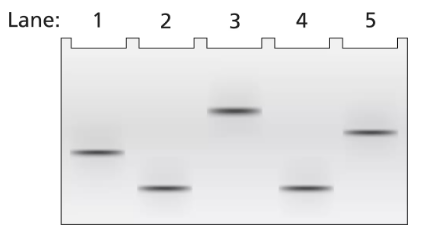
A 2-kb fragment of E. coli DNA contains the complete sequence of a gene for which transcription is terminated by the rho protein. The fragment contains the complete promoter sequence as well as the terminator region of the gene. The cloned fragment is examined by band shift assay. Each lane of a single electrophoresis gel contains the 2-kb cloned fragment under the following conditions:
Lane 1: 2-kb fragment alone
Lane 2: 2-kb fragment plus the core enzyme
Lane 3: 2-kb fragment plus the RNA polymerase holoenzyme
Lane 4: 2-kb fragment plus rho protein
Diagram the relative positions expected for the DNA fragments in this gel electrophoresis analysis.
Problem 17b
A 2-kb fragment of E. coli DNA contains the complete sequence of a gene for which transcription is terminated by the rho protein. The fragment contains the complete promoter sequence as well as the terminator region of the gene. The cloned fragment is examined by band shift assay. Each lane of a single electrophoresis gel contains the 2-kb cloned fragment under the following conditions:
Lane 1: 2-kb fragment alone
Lane 2: 2-kb fragment plus the core enzyme
Lane 3: 2-kb fragment plus the RNA polymerase holoenzyme
Lane 4: 2-kb fragment plus rho protein
Explain the relative positions of bands in lanes 1 and 3.
Problem 17c
A 2-kb fragment of E. coli DNA contains the complete sequence of a gene for which transcription is terminated by the rho protein. The fragment contains the complete promoter sequence as well as the terminator region of the gene. The cloned fragment is examined by band shift assay. Each lane of a single electrophoresis gel contains the 2-kb cloned fragment under the following conditions:
Lane 1: 2-kb fragment alone
Lane 2: 2-kb fragment plus the core enzyme
Lane 3: 2-kb fragment plus the RNA polymerase holoenzyme
Lane 4: 2-kb fragment plus rho protein
Explain the relative positions of bands in lanes 1 and 4.
Problem 18a
A 3.5-kb segment of DNA containing the complete sequence of a mouse gene is available. The DNA segment contains the promoter sequence and extends beyond the polyadenylation site of the gene. The DNA is studied by band shift assay, and the following gel bands are observed.
Match these conditions to a specific lane of the gel.
3.5-kb fragment plus TFIIB and TFIID
Problem 18b
A 3.5-kb segment of DNA containing the complete sequence of a mouse gene is available. The DNA segment contains the promoter sequence and extends beyond the polyadenylation site of the gene. The DNA is studied by band shift assay, and the following gel bands are observed.
Match these conditions to a specific lane of the gel.
3.5-kb fragment plus TFIIB, TFIID, TFIIF, and RNA polymerase II
Problem 18c
A 3.5-kb segment of DNA containing the complete sequence of a mouse gene is available. The DNA segment contains the promoter sequence and extends beyond the polyadenylation site of the gene. The DNA is studied by band shift assay, and the following gel bands are observed.
Match these conditions to a specific lane of the gel.
3.5-kb fragment alone
Problem 18d
A 3.5-kb segment of DNA containing the complete sequence of a mouse gene is available. The DNA segment contains the promoter sequence and extends beyond the polyadenylation site of the gene. The DNA is studied by band shift assay, and the following gel bands are observed.
Match these conditions to a specific lane of the gel.
3.5-kb fragment plus RNA polymerase II
Problem 18e
A 3.5-kb segment of DNA containing the complete sequence of a mouse gene is available. The DNA segment contains the promoter sequence and extends beyond the polyadenylation site of the gene. The DNA is studied by band shift assay, and the following gel bands are observed.
Match these conditions to a specific lane of the gel.
3.5-kb fragment plus TFIIB
Problem 19a
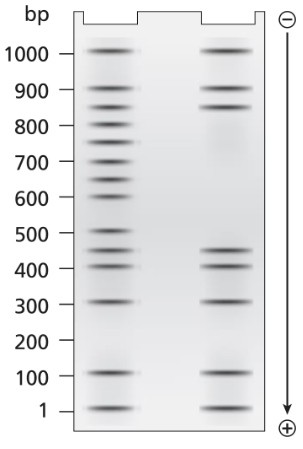
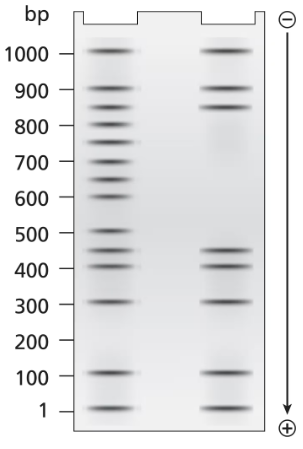
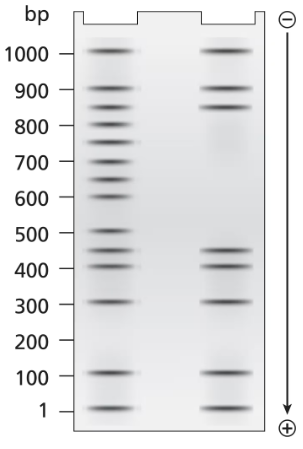
A 1.0-kb DNA fragment from the end of the mouse gene described in the previous problem is examined by DNA footprint protection analysis. Two samples are end-labeled with ³²P and one of the two is mixed with TFIIB, TFIID, and RNA polymerase II. The DNA exposed to these proteins is run in the right-hand lane of the gel shown below and the control DNA is run in the left-hand. Both DNA samples are treated with DNase I before running the samples on the electrophoresis gel.
What length of DNA is bound by the transcriptional proteins? Explain how the gel results support this interpretation.
Problem 19b
A 1.0-kb DNA fragment from the end of the mouse gene described in the previous problem is examined by DNA footprint protection analysis. Two samples are end-labeled with ³²P, and one of the two is mixed with TFIIB, TFIID, and RNA polymerase II. The DNA exposed to these proteins is run in the right-hand lane of the gel shown below and the control DNA is run in the left-hand. Both DNA samples are treated with DNase I before running the samples on the electrophoresis gel.
Draw a diagram of this DNA fragment bound by the transcriptional proteins, showing the approximate position of proteins along the fragment.
Problem 19c
A 1.0-kb DNA fragment from the end of the mouse gene described in the previous problem is examined by DNA footprint protection analysis. Two samples are end-labeled with ³²P and one of the two is mixed with TFIIB, TFIID, and RNA polymerase II. The DNA exposed to these proteins is run in the right-hand lane of the gel shown below and the control DNA is run in the left-hand. Both DNA samples are treated with DNase I before running the samples on the electrophoresis gel.
Explain the role of DNase I.
Problem 20a
Wild-type E. coli grow best at 37°C but can grow efficiently up to 42°C. An E. coli strain has a mutation of the sigma subunit that results in an RNA polymerase holoenzyme that is stable and transcribes at wild-type levels at 37°C. The mutant holoenzyme is progressively destabilized as the temperature is raised, and it completely denatures and ceases to carry out transcription at 42°C. Relative to wild-type growth, characterize the ability of the mutant strain to carry out transcription at 37°C
Problem 20b
Wild-type E. coli grows best at 37°C but can grow efficiently up to 42°C. An E. coli strain has a mutation of the sigma subunit that results in an RNA polymerase holoenzyme that is stable and transcribes at wild-type levels at 37°C. The mutant holoenzyme is progressively destabilized as the temperature is raised, and it completely denatures and ceases to carry out transcription at 42°C. Relative to wild-type growth, characterize the ability of the mutant strain to carry out transcription at 40°C
Problem 20c
Wild-type E. coli grows best at 37°C but can grow efficiently up to 42°C. An E. coli strain has a mutation of the sigma subunit that results in an RNA polymerase holoenzyme that is stable and transcribes at wild-type levels at 37°C. The mutant holoenzyme is progressively destabilized as the temperature is raised, and it completely denatures and ceases to carry out transcription at 42°C. Relative to wild-type growth, characterize the ability of the mutant strain to carry out transcription at 42°C
Problem 20d
Wild-type E. coli grows best at 37°C but can grow efficiently up to 42°C. An E. coli strain has a mutation of the sigma subunit that results in an RNA polymerase holoenzyme that is stable and transcribes at wild-type levels at 37°C. The mutant holoenzyme is progressively destabilized as the temperature is raised, and it completely denatures and ceases to carry out transcription at 42°C. Relative to wild-type growth, characterize the ability of the mutant strain to carry out transcription at What term best characterizes the type of mutation exhibited by the mutant bacterial strain? (Hint: The term was used in Chapter 4 to describe the Himalayan allele of the mammalian C gene.)
Problem 21a
A mutant strain of Salmonella bacteria carries a mutation of the rho protein that has full activity at 37°C but is completely inactivated when the mutant strain is grown at 40°C. Speculate about the kind of differences you would expect to see if you compared a broad spectrum of mRNAs from the mutant strain grown at 37°C and the same spectrum of mRNAs from the strain when grown at 40°C.
Problem 21b
A mutant strain of Salmonella bacteria carries a mutation of the rho protein that has full activity at 37°C but is completely inactivated when the mutant strain is grown at 40°C. Are all mRNAs affected by the rho protein mutation in the same way? Why or why not?
Problem 22a
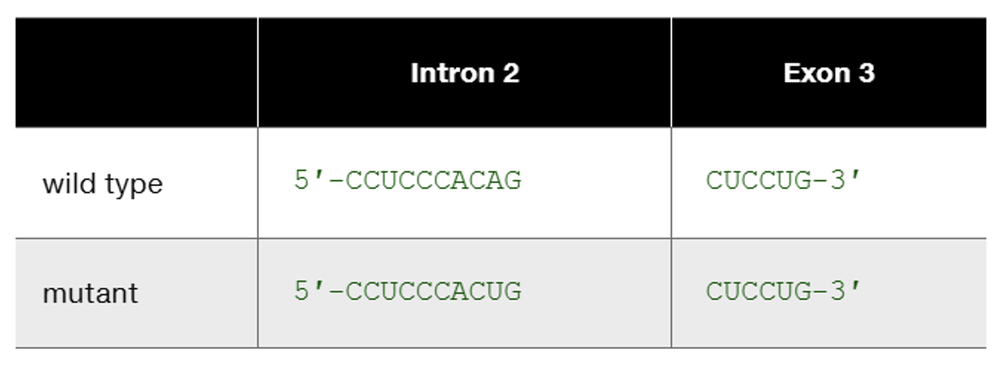
The human β-globin wild-type allele and a certain mutant allele are identical in sequence except for a single base-pair substitution that changes one nucleotide at the end of intron 2. The wild-type and mutant sequences of the affected portion of pre-mRNA are
Speculate about the way in which this base substitution causes mutation of β-globin protein.
Problem 22b
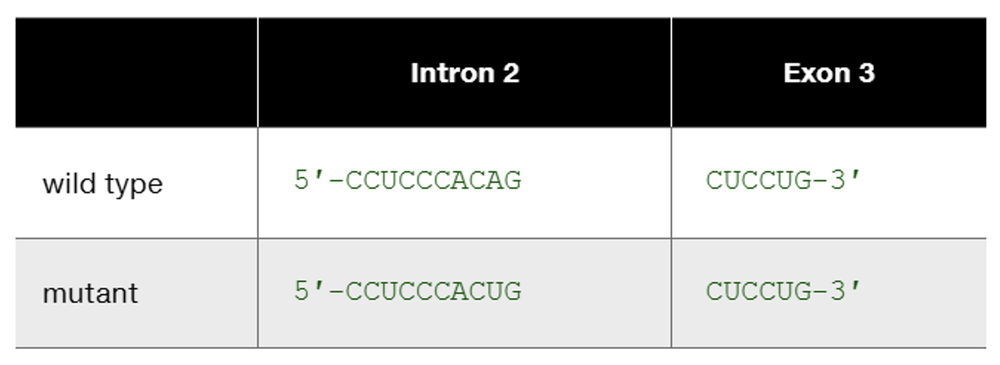
The human β-globin wild-type allele and a certain mutant allele are identical in sequence except for a single base-pair substitution that changes one nucleotide at the end of intron 2. The wild-type and mutant sequences of the affected portion of pre-mRNA are
This is one example of how DNA sequence change occurring somewhere other than in an exon can produce mutation. List other kinds of DNA sequence changes occurring outside exons that can produce mutation. In each case, characterize the kind of change you would expect to see in mutant mRNA or mutant protein.
Problem 23a
Microbiologists describe the processes of transcription and translation as 'coupled' in bacteria. This term indicates that a bacterial mRNA can be undergoing transcription at the same moment it is also undergoing translation.
How is coupling of transcription and translation possible in bacteria?
Problem 24a
A full-length eukaryotic gene is inserted into a bacterial chromosome. The gene contains a complete promoter sequence and a functional polyadenylation sequence, and it has wild-type nucleotides throughout the transcribed region. However, the gene fails to produce a functional protein. List at least three possible reasons why this eukaryotic gene is not expressed in bacteria.
Problem 24b
A full-length eukaryotic gene is inserted into a bacterial chromosome. The gene contains a complete promoter sequence and a functional polyadenylation sequence, and it has wild-type nucleotides throughout the transcribed region. However, the gene fails to produce a functional protein. What changes would you recommend to permit expression of this eukaryotic gene in a bacterial cell?
Problem 25a
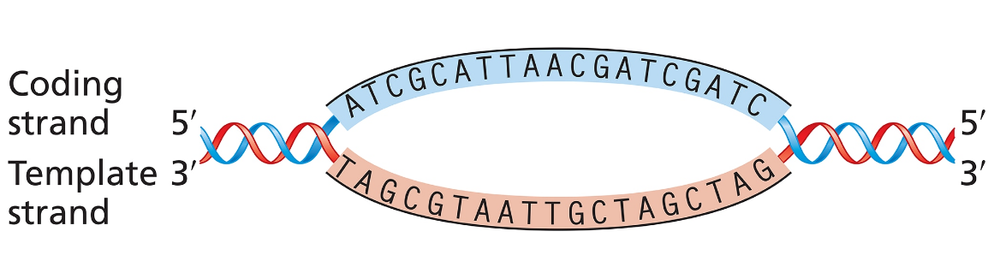
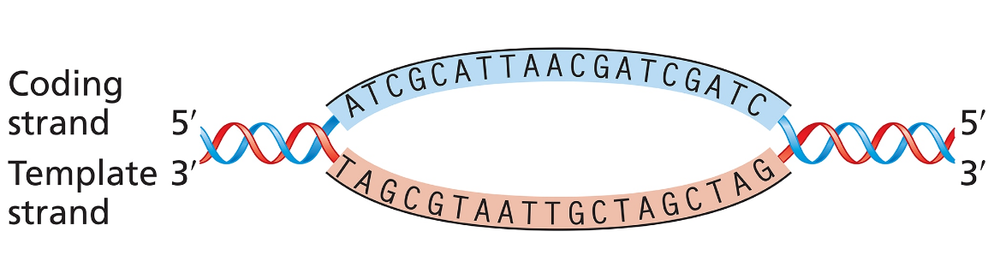
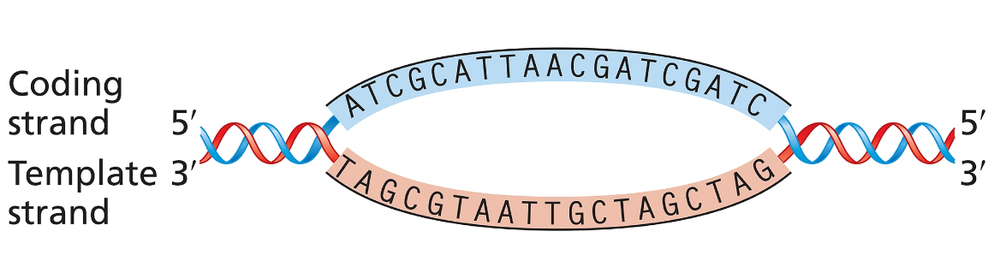
The accompanying illustration shows a portion of a gene undergoing transcription. The template and coding strands for the gene are labeled, and a segment of DNA sequence is given.
For this gene segment, superimpose a drawing of RNA polymerase as it nears the end of transcription of the DNA sequence.
Problem 25b
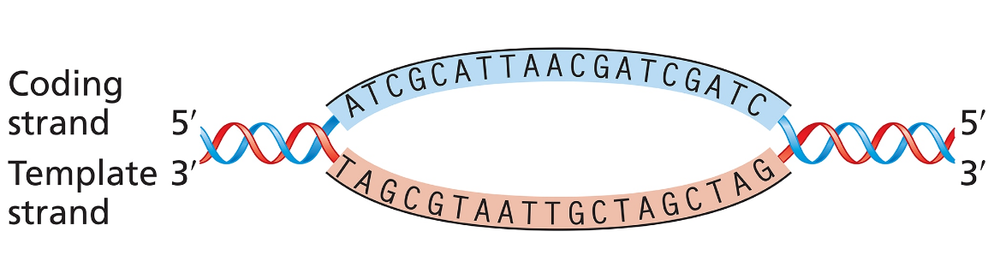
The accompanying illustration shows a portion of a gene undergoing transcription. The template and coding strands for the gene are labeled, and a segment of DNA sequence is given.
For this gene segment indicate the direction in which RNA polymerase moves as it transcribes this gene.
Problem 25c
The accompanying illustration shows a portion of a gene undergoing transcription. The template and coding strands for the gene are labeled, and a segment of DNA sequence is given.
For this gene segment, write the polarity and sequence [TIP 1] of the RNA transcript from the DNA sequence given.
Problem 25d
The accompanying illustration shows a portion of a gene undergoing transcription. The template and coding strands for the gene are labeled, and a segment of DNA sequence is given.
For this gene segment identify the direction in which the promoter [TIP 2] for this gene is located.
Problem 26a
DNA footprint protection is a method that determines whether proteins bind to a specific sample of DNA and thus protect part of the DNA from random enzymatic cleavage by DNase I. A 400-bp segment of cloned DNA is thought to contain a promoter. The cloned DNA is analyzed by DNA footprinting to help determine if it has the capacity to act as a promoter sequence. The accompanying gel has two lanes, each containing the cloned 400-bp DNA fragment treated with DNase I to randomly cleave unprotected DNA. Lane 1 is cloned DNA that was mixed with RNA polymerase II and several TFII transcription factors before exposure to DNase I. Lane 2 contains cloned DNA that was exposed only to DNase I. RNA pol II and TFIIs were not mixed with that DNA before adding DNase I. Explain why this gel provides evidence that the cloned DNA may act as a promoter sequence.
Problem 26b
DNA footprint protection is a method that determines whether proteins bind to a specific sample of DNA and thus protect part of the DNA from random enzymatic cleavage by DNase I. A 400-bp segment of cloned DNA is thought to contain a promoter. The cloned DNA is analyzed by DNA footprinting to help determine if it has the capacity to act as a promoter sequence. The accompanying gel has two lanes, each containing the cloned 400-bp DNA fragment treated with DNase I to randomly cleave unprotected DNA. Lane 1 is cloned DNA that was mixed with RNA polymerase II and several TFII transcription factors before exposure to DNase I. Lane 2 contains cloned DNA that was exposed only to DNase I. RNA pol II and TFIIs were not mixed with that DNA before adding DNase I. Approximately what length is the DNA region protected by RNA pol II and TFIIs?
Problem 26c
DNA footprint protection is a method that determines whether proteins bind to a specific sample of DNA and thus protect part of the DNA from random enzymatic cleavage by DNase I. A 400-bp segment of cloned DNA is thought to contain a promoter. The cloned DNA is analyzed by DNA footprinting to help determine if it has the capacity to act as a promoter sequence. The accompanying gel has two lanes, each containing the cloned 400-bp DNA fragment treated with DNase I to randomly cleave unprotected DNA. Lane 1 is cloned DNA that was mixed with RNA polymerase II and several TFII transcription factors before exposure to DNase I. Lane 2 contains cloned DNA that was exposed only to DNase I. RNA pol II and TFIIs were not mixed with that DNA before adding DNase I. What additional genetic experiments would you suggest to verify that this region of cloned DNA contains a functional promoter?
Problem 27
Suppose you have a 1-kb segment of cloned DNA that is suspected to contain a eukaryotic promoter, including a TATA box, a CAAT box, and an upstream GC-rich sequence. The clone also contains a gene whose transcript is readily detectable. Your laboratory supervisor asks you to outline an experiment that will (1) determine if eukaryotic transcription factors (TF) bind to the fragment and, if so, (2) identify where on the fragment the transcription factors bind. All necessary reagents, equipment, and experimental know-how are available in the laboratory. Your assignment is to propose techniques to be used to address the two items your supervisor has listed and to describe the kind of results that would indicate binding of TF to the DNA and the location of the binding.