Which gas sample has the greatest pressure? Assume that all the samples are at the same temperature. Explain.
Ch.6 - Gases

Chapter 6, Problem 56
This picture represents a sample of gas at a pressure of 1 atm, a volume of 1 L, and a temperature of 25 °C. Draw a similar picture showing what would happen to the sample if the volume were reduced to 0.5 L and the temperature were increased to 250 °C. What would happen to the pressure?

 Verified step by step guidance
Verified step by step guidance1
Step 1: Identify the initial conditions of the gas sample: pressure (P1) = 1 atm, volume (V1) = 1 L, and temperature (T1) = 25 °C (which is 298 K).
Step 2: Convert the final temperature to Kelvin: T2 = 250 °C + 273 = 523 K.
Step 3: Use the combined gas law to relate the initial and final states of the gas: (P1 * V1) / T1 = (P2 * V2) / T2.
Step 4: Substitute the known values into the combined gas law equation: (1 atm * 1 L) / 298 K = (P2 * 0.5 L) / 523 K.
Step 5: Solve for the final pressure (P2) by isolating P2 in the equation.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
1mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Ideal Gas Law
The Ideal Gas Law is a fundamental equation in chemistry that relates the pressure (P), volume (V), temperature (T), and number of moles (n) of a gas. It is expressed as PV = nRT, where R is the ideal gas constant. This law helps predict how changing one of these variables affects the others, making it essential for understanding gas behavior under different conditions.
Recommended video:
Guided course

Ideal Gas Law Formula
Charles's Law
Charles's Law states that the volume of a gas is directly proportional to its temperature when pressure is held constant. This means that if the temperature increases, the volume must also increase, and vice versa. In the context of the question, reducing the volume while increasing the temperature will significantly affect the pressure of the gas.
Recommended video:
Guided course
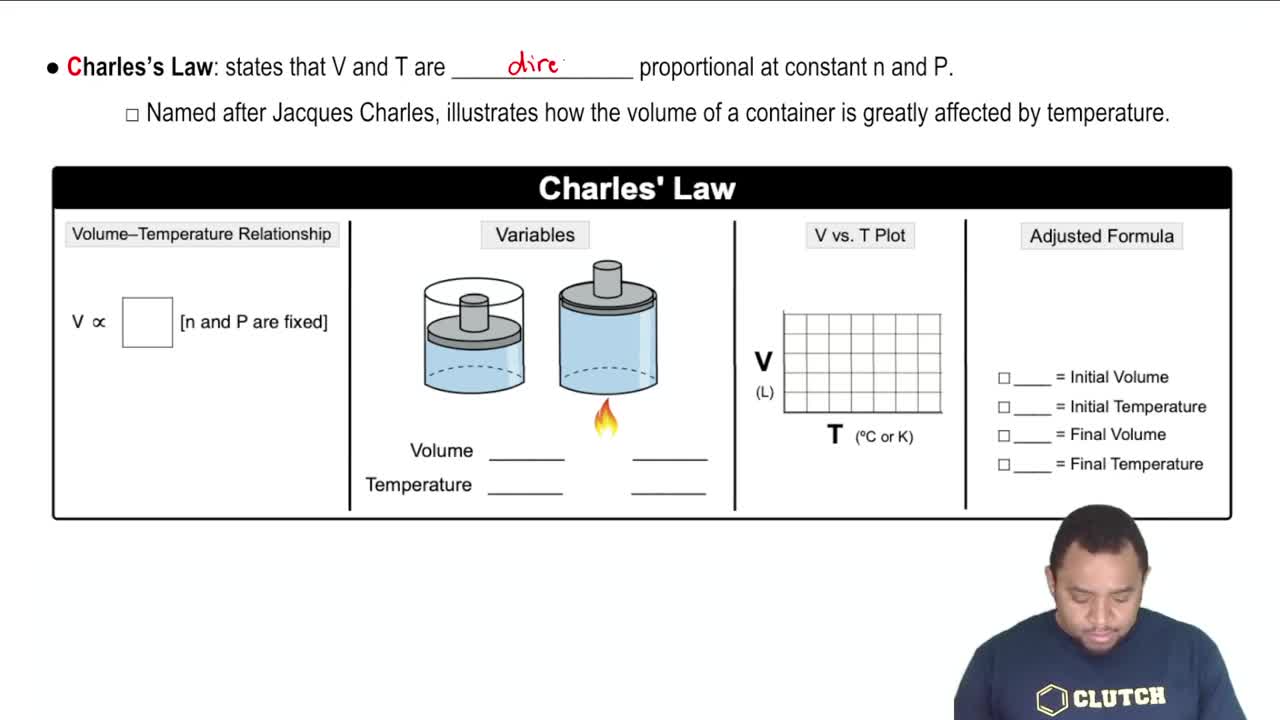
Charles's Law
Boyle's Law
Boyle's Law describes the inverse relationship between the pressure and volume of a gas at constant temperature. According to this law, if the volume of a gas decreases, the pressure increases, provided the temperature remains constant. This principle is crucial for understanding how the pressure of the gas sample will change when its volume is halved.
Recommended video:
Guided course
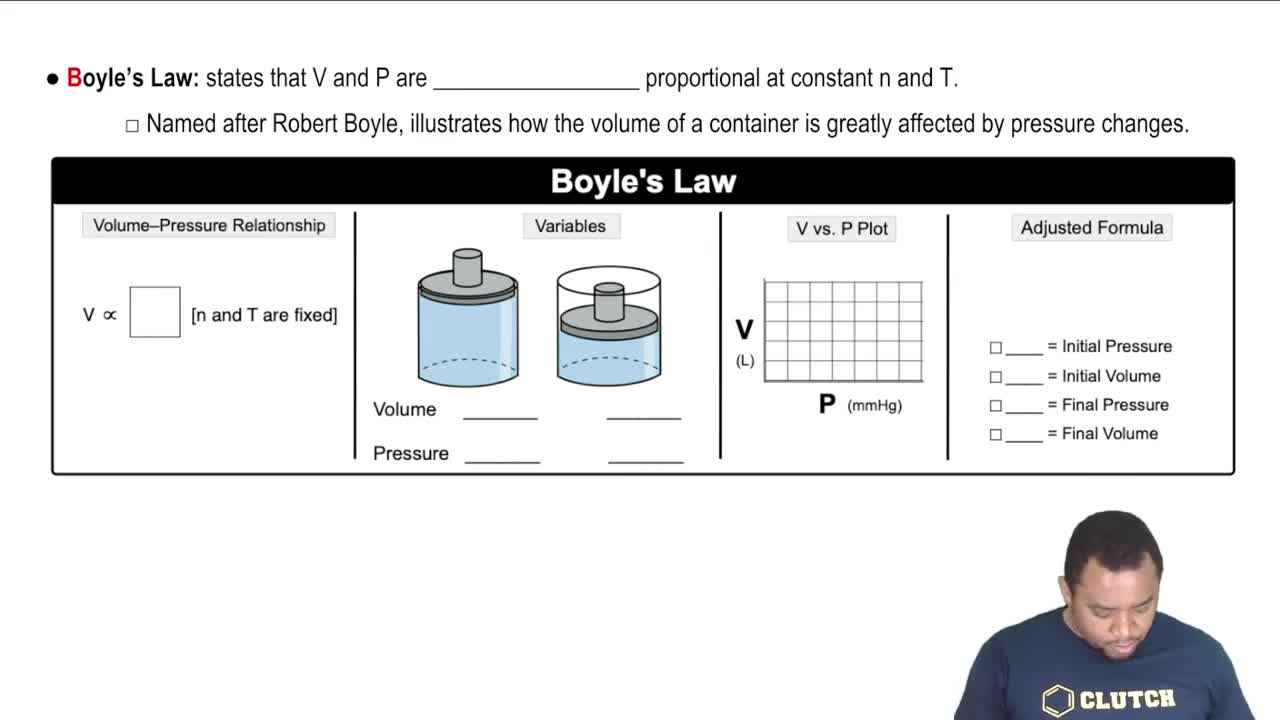
Boyle's Law
Related Practice
Textbook Question
Textbook Question
Write balanced complete ionic and net ionic equations for each reaction. a. HCl(aq) + LiOH(aq) → H2O(l) + LiCl(aq)
Textbook Question
Aerosol cans carry clear warnings against incineration because of the high pressures that can develop upon heating. Suppose that a can contains a residual amount of gas at a pressure of 755 mmHg and a temperature of 25 °C. What would the pressure be if the can were heated to 1155 °C?
1
views
Textbook Question
A sample of nitrogen gas in a 1.75-L container exerts a pressure of 1.35 atm at 25 °C. What is the pressure if the volume of the container is maintained constant and the temperature is raised to 355 °C?
Textbook Question
Use the molar volume of a gas at STP to determine the volume (in L) occupied by 27.2 g of argon at STP.
