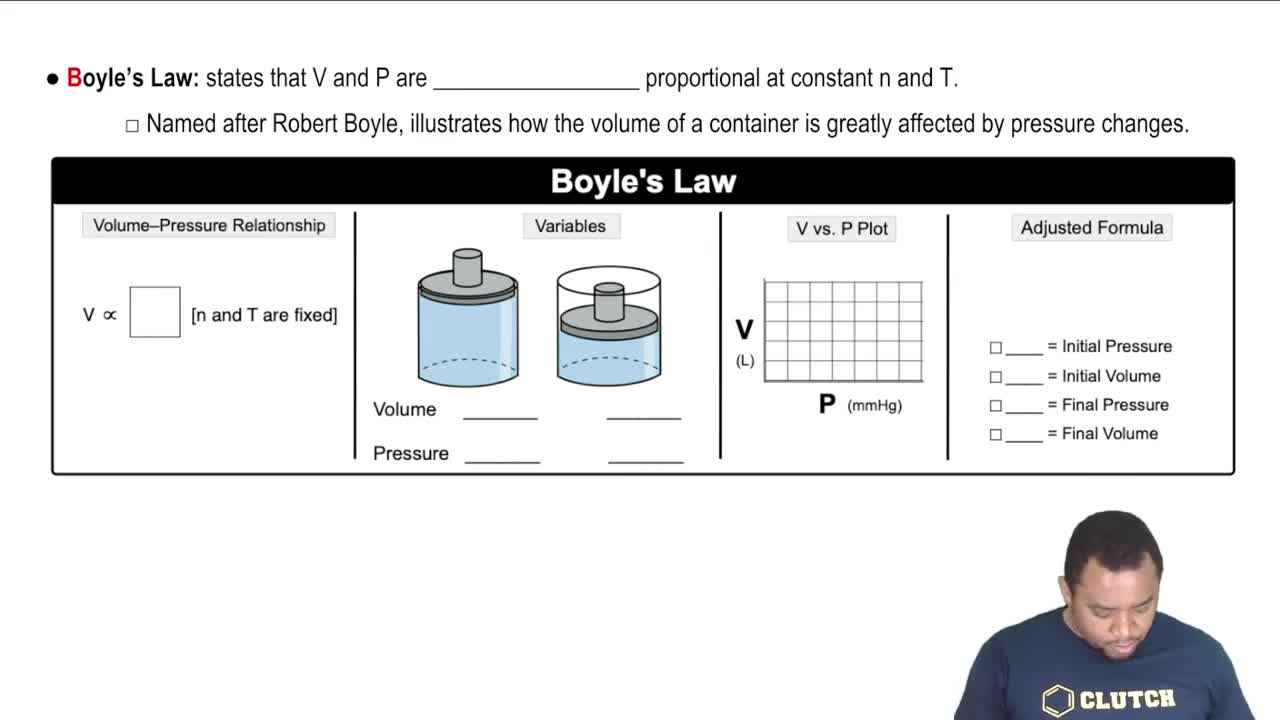
Given a barometric pressure of 751.5 mmHg, calculate the pressure of each gas sample as indicated by the manometer.
(a)

 Verified step by step guidance
Verified step by step guidance

Given a barometric pressure of 751.5 mmHg, calculate the pressure of each gas sample as indicated by the manometer.
(a)
Given a barometric pressure of 751.5 mmHg, calculate the pressure of each gas sample as indicated by the manometer.
(b)
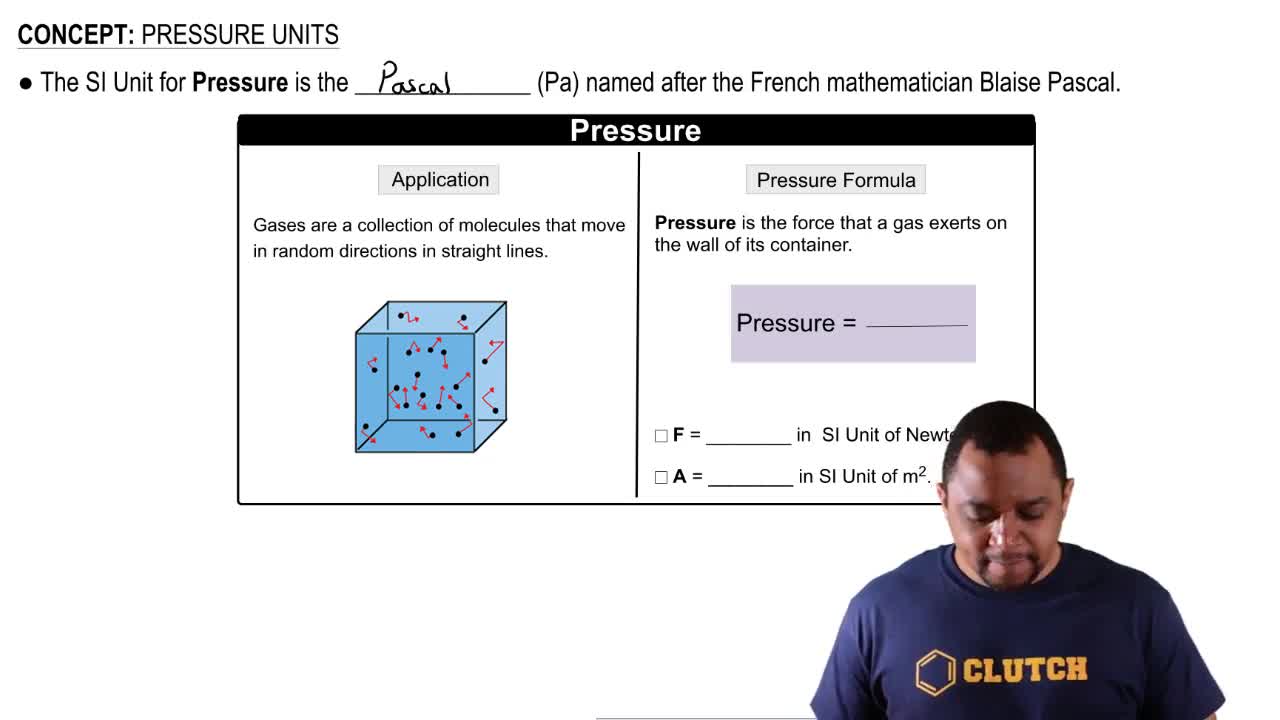
A sample of gas has an initial volume of 4.20 L at a pressure of 755 mmHg. If the volume of the gas is increased to 7.10 L (at constant temperature), what is its pressure?
A 48.3-mL sample of gas in a cylinder is warmed from 22 °C to 87 °C. What is its volume at the final temperature?
A syringe containing 1.55 mL of oxygen gas is cooled from 95.3 °C to 0.0 °C. What is the final volume of oxygen gas?
A balloon contains 0.119 mol of gas and has a volume of 3.21 L. If an additional 0.103 mol of gas is added to the balloon (at constant temperature and pressure), what is its final volume?