Calculate the mass (in kilograms) of chlorine in 25 kg of each chlorofluorocarbon (CFC). a. CF2Cl2 c. C2F3Cl3 d. CF3Cl
Ch.3 - Molecules and Compounds

Chapter 3, Problem 94
How many fluorine atoms are present in 45.3 g of C2F4?
 Verified step by step guidance
Verified step by step guidance1
Calculate the molar mass of \( \text{C}_2\text{F}_4 \) by adding the atomic masses of its constituent atoms: 2 carbon atoms and 4 fluorine atoms.
Determine the number of moles of \( \text{C}_2\text{F}_4 \) in 45.3 g by using the formula: \( \text{moles} = \frac{\text{mass}}{\text{molar mass}} \).
Use Avogadro's number \( (6.022 \times 10^{23} \text{ molecules/mol}) \) to find the number of molecules of \( \text{C}_2\text{F}_4 \) in the calculated moles.
Recognize that each molecule of \( \text{C}_2\text{F}_4 \) contains 4 fluorine atoms.
Multiply the number of \( \text{C}_2\text{F}_4 \) molecules by 4 to find the total number of fluorine atoms.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
2mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Molar Mass
Molar mass is the mass of one mole of a substance, typically expressed in grams per mole (g/mol). For C2F4, the molar mass is calculated by summing the atomic masses of its constituent elements: carbon (C) and fluorine (F). This value is essential for converting between grams and moles, which is necessary to determine the number of atoms in a given mass of the compound.
Recommended video:
Guided course

Molar Mass Concept
Avogadro's Number
Avogadro's number, approximately 6.022 x 10^23, is the number of particles (atoms, molecules, etc.) in one mole of a substance. This constant allows chemists to relate the macroscopic scale of substances (grams) to the microscopic scale (individual atoms or molecules). In this problem, it will be used to find the total number of fluorine atoms once the moles of C2F4 are determined.
Recommended video:
Guided course
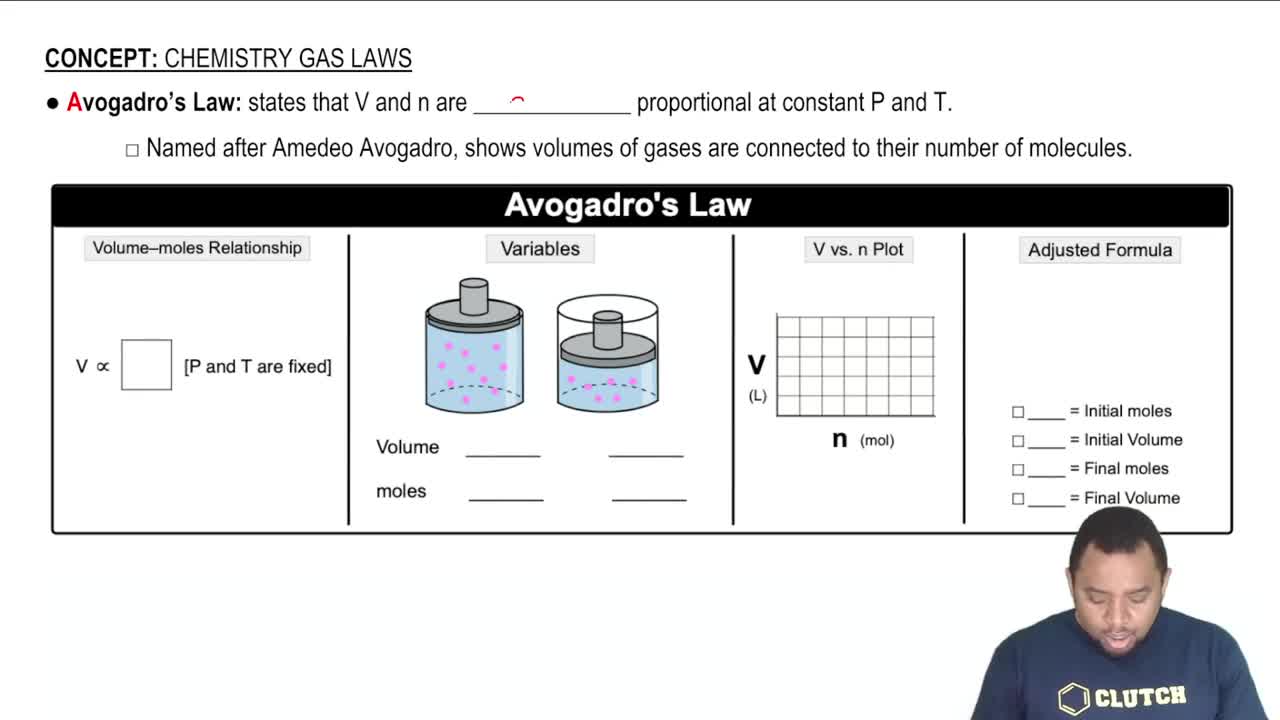
Avogadro's Law
Stoichiometry
Stoichiometry is the calculation of reactants and products in chemical reactions based on the conservation of mass. In the context of C2F4, stoichiometry helps determine the ratio of fluorine atoms to the moles of C2F4. Since each molecule of C2F4 contains four fluorine atoms, understanding this relationship is crucial for calculating the total number of fluorine atoms present in the given mass.
Recommended video:
Guided course

Stoichiometry Concept
Related Practice
Textbook Question
1
views
Textbook Question
Calculate the mass (in kilograms) of chlorine in 25 kg of each chlorofluorocarbon (CFC). b. CFCl3
1
views
Textbook Question
How many bromine atoms are present in 12.8 g of CH2Br2?
Textbook Question
A chemist decomposes samples of several compounds; the masses of their constituent elements are listed. Calculate the empirical formula for each compound. a. 1.245 g Ni, 5.381 g I
Textbook Question
A chemist decomposes samples of several compounds; the masses of their constituent elements are listed. Calculate the empirical formula for each compound. b. 2.677 g Ba, 3.115 g Br
Textbook Question
A chemist decomposes samples of several compounds; the masses of their constituent elements are listed. Calculate the empirical formula for each compound. c. 2.128 g Be, 7.557 g S, 15.107 g O
1
views
