Calculate the mass (in kilograms) of chlorine in 25 kg of each chlorofluorocarbon (CFC). b. CFCl3
Ch.3 - Molecules and Compounds

Chapter 3, Problem 96a
A chemist decomposes samples of several compounds; the masses of their constituent elements are listed. Calculate the empirical formula for each compound. a. 1.245 g Ni, 5.381 g I
 Verified step by step guidance
Verified step by step guidance1
Determine the number of moles of each element by dividing the mass of each element by its molar mass. For Nickel (Ni), use its molar mass of approximately 58.69 g/mol, and for Iodine (I), use its molar mass of approximately 126.90 g/mol.
Calculate the moles of Nickel (Ni) using the formula: \( \text{moles of Ni} = \frac{1.245 \text{ g}}{58.69 \text{ g/mol}} \).
Calculate the moles of Iodine (I) using the formula: \( \text{moles of I} = \frac{5.381 \text{ g}}{126.90 \text{ g/mol}} \).
Determine the simplest whole number ratio of moles of Ni to moles of I by dividing each by the smallest number of moles calculated in the previous steps.
Write the empirical formula by using the whole number ratio as subscripts for each element in the compound.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
2mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Empirical Formula
The empirical formula of a compound represents the simplest whole-number ratio of the elements present in that compound. It is derived from the mass of each element and is crucial for understanding the composition of the compound. For example, if a compound contains 1 mole of element A and 2 moles of element B, its empirical formula would be AB2.
Recommended video:
Guided course
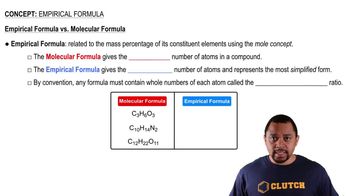
Empirical vs Molecular Formula
Molar Mass
Molar mass is the mass of one mole of a substance, typically expressed in grams per mole (g/mol). It is essential for converting the mass of each element in a sample to moles, which allows for the determination of the ratio of elements in the empirical formula. For instance, the molar mass of nickel (Ni) is approximately 58.69 g/mol, and for iodine (I), it is about 126.90 g/mol.
Recommended video:
Guided course

Molar Mass Concept
Stoichiometry
Stoichiometry is the branch of chemistry that deals with the quantitative relationships between the reactants and products in a chemical reaction. It involves using balanced chemical equations to calculate the amounts of substances involved. In the context of finding an empirical formula, stoichiometry helps in determining the mole ratios of the elements based on their masses.
Recommended video:
Guided course

Stoichiometry Concept
Related Practice
Textbook Question
1
views
Textbook Question
How many bromine atoms are present in 12.8 g of CH2Br2?
Textbook Question
How many fluorine atoms are present in 45.3 g of C2F4?
Textbook Question
A chemist decomposes samples of several compounds; the masses of their constituent elements are listed. Calculate the empirical formula for each compound. b. 2.677 g Ba, 3.115 g Br
Textbook Question
A chemist decomposes samples of several compounds; the masses of their constituent elements are listed. Calculate the empirical formula for each compound. c. 2.128 g Be, 7.557 g S, 15.107 g O
1
views
Textbook Question
Calculate the empirical formula for each stimulant based on its elemental mass percent composition.
a. nicotine (found in tobacco leaves): C 74.03%, H 8.70%, N 17.27%
b. caffeine (found in coffee beans): C 49.48%, H 5.19%, N 28.85%, O 16.48%
3
views
