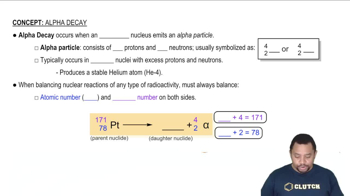
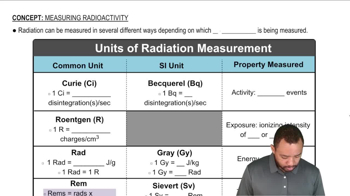
Alpha decay
Alpha decay is a type of radioactive decay in which an unstable nucleus emits an alpha particle, consisting of two protons and two neutrons. This process reduces the atomic number of the element by two, transforming it into a different element. In the context of Polonium-218, each decay event results in the emission of an alpha particle, which is essential for calculating the total number of emissions over a given time frame.

 Verified step by step guidance
Verified step by step guidance