Silicon has three naturally occurring isotopes (Si-28, Si-29, and Si-30). The mass and natural abundance of Si-28 are 27.9769 amu and 92.2%, respectively. The mass and natural abundance of Si-29 are 28.9765 amu and 4.67%, respectively. Find the mass and natural abundance of Si-30.
Ch.2 - Atoms & Elements

Chapter 2, Problem 84
An element has four naturally occurring isotopes with the masses and natural abundances given here. Find the atomic mass of the element and identify it.
Isotope Mass (amu) Abundance (%)
1 135.90714 0.19
2 137.90599 0.25
3 139.90543 88.43
4 141.90924 11.13
 Verified step by step guidance
Verified step by step guidance1
First, we need to understand that the atomic mass of an element is the weighted average of the masses of its isotopes. The weight of each isotope is its abundance. So, we will multiply the mass of each isotope by its abundance to get a 'weighted' mass.
Next, convert the abundances from percentages to fractions by dividing each by 100. For example, the abundance of the first isotope would be 0.19/100 = 0.0019.
Then, multiply the mass of each isotope by its corresponding abundance (now in fraction form). For example, the weighted mass of the first isotope would be 135.90714 * 0.0019.
Add up all the weighted masses. This will give you the atomic mass of the element.
Finally, compare the calculated atomic mass with the atomic masses of known elements to identify the element. You can use a periodic table for this comparison.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
4mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Isotopes
Isotopes are variants of a chemical element that have the same number of protons but different numbers of neutrons, resulting in different atomic masses. For example, carbon has isotopes like carbon-12 and carbon-14. Understanding isotopes is crucial for calculating the average atomic mass of an element based on its isotopic composition.
Recommended video:
Guided course

Isotopes
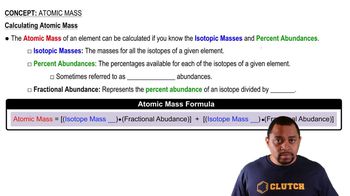
Atomic Mass Calculation
The atomic mass of an element is calculated by taking the weighted average of the masses of its isotopes, considering their natural abundances. This is done using the formula: Atomic Mass = (mass1 × abundance1) + (mass2 × abundance2) + ... + (massn × abundancen). This concept is essential for determining the overall atomic mass from the provided isotopic data.
Recommended video:
Guided course
Calculating Atomic Mass
Natural Abundance
Natural abundance refers to the relative percentage of each isotope of an element found in nature. It is expressed as a percentage and is critical for accurately calculating the average atomic mass. In the given question, the abundances of the isotopes must be used to weight their respective masses in the atomic mass calculation.
Recommended video:
Guided course
Nature of Energy
Related Practice
Textbook Question
3
views
Textbook Question
The atomic mass of fluorine is 18.998 amu, and its mass spectrum shows a large peak at this mass. The atomic mass of chlorine is 35.45 amu, yet the mass spectrum of chlorine does not show a peak at this mass. Explain the difference.
Textbook Question
An element has two naturally occurring isotopes. Isotope 1 has a mass of 120.9038 amu and a relative abundance of 57.4%, and isotope 2 has a mass of 122.9042 amu. Find the atomic mass of this element and identify it.
3
views
Textbook Question
Bromine has two naturally occurring isotopes (Br-79 and Br-81) and has an atomic mass of 79.904 amu. The mass of Br-81 is 80.9163 amu, and its natural abundance is 49.31%. Calculate the mass and natural abundance of Br-79.
4
views
Textbook Question
Use the mass spectrum of europium to determine the atomic mass of europium.
1
views
