Textbook Question
Balance each redox reaction occurring in basic aqueous solution. c. NO2–(aq) + Al(s) → NH3(g) + AlO2–(aq)


 Verified step by step guidance
Verified step by step guidance

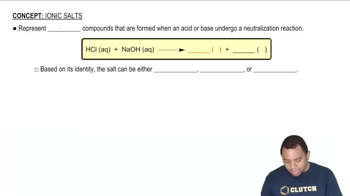
Balance each redox reaction occurring in basic aqueous solution. c. NO2–(aq) + Al(s) → NH3(g) + AlO2–(aq)
Sketch a voltaic cell for each redox reaction. Label the anode and cathode and indicate the half-reaction that occurs at each electrode and the species present in each solution. Also indicate the direction of electron flow. a. Ni2+(aq) + Mg(s) → Ni(s) + Mg2+(aq)
Calculate the standard cell potential for each of the electro- chemical cells in Problem 43.
Use line notation to represent each electrochemical cell in Problem 43.
Make a sketch of the voltaic cell represented by the line notation. Write the overall balanced equation for the reaction and calculate E°cell. Sn(s) | Sn2+(aq) || NO(g) | NO3–(aq), H+(aq) | Pt(s)