Balance each redox reaction occurring in basic aqueous solution. b. Al(s) + MnO4–(aq) → MnO2(s) + Al(OH)4–(aq)
Ch.20 - Electrochemistry

Chapter 20, Problem 42c
Balance each redox reaction occurring in basic aqueous solution. c. NO2–(aq) + Al(s) → NH3(g) + AlO2–(aq)
 Verified step by step guidance
Verified step by step guidance1
Identify the oxidation and reduction half-reactions. In this case, NO_2^- is reduced to NH_3, and Al is oxidized to AlO_2^-.
Write the reduction half-reaction: NO_2^- \rightarrow NH_3. Balance the atoms other than O and H first, then balance O by adding H_2O, and H by adding H^+. Finally, balance the charge by adding electrons.
Write the oxidation half-reaction: Al \rightarrow AlO_2^-. Balance the atoms other than O and H first, then balance O by adding H_2O, and H by adding H^+. Finally, balance the charge by adding electrons.
Since the solution is basic, add OH^- to both sides of each half-reaction to neutralize the H^+ ions, forming water. Simplify the water molecules if necessary.
Combine the balanced half-reactions, ensuring that the electrons cancel out, to obtain the balanced overall redox reaction.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
13mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Redox Reactions
Redox reactions, or reduction-oxidation reactions, involve the transfer of electrons between two species. In these reactions, one species is oxidized (loses electrons) while the other is reduced (gains electrons). Understanding the oxidation states of the elements involved is crucial for identifying which species undergo oxidation and reduction.
Recommended video:
Guided course
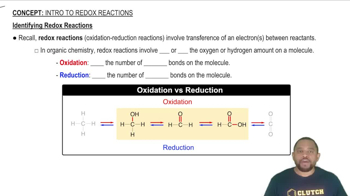
Identifying Redox Reactions
Balancing Redox Reactions in Basic Solution
Balancing redox reactions in basic solutions requires a specific approach. First, the half-reactions for oxidation and reduction are balanced separately. Then, hydroxide ions (OH-) are added to neutralize any hydrogen ions (H+) produced, ensuring that the reaction adheres to the basic conditions. Finally, the overall reaction is combined and simplified.
Recommended video:
Guided course

Balancing Basic Redox Reactions
Half-Reaction Method
The half-reaction method is a systematic way to balance redox reactions by separating the oxidation and reduction processes. Each half-reaction is balanced for mass and charge, allowing for a clear understanding of electron transfer. This method is particularly useful in complex reactions, as it simplifies the balancing process by focusing on one half of the reaction at a time.
Recommended video:
Guided course
Method 1 of Radioactive Half-Life
Related Practice
Textbook Question
Textbook Question
Balance each redox reaction occurring in basic aqueous solution. a. MnO4–(aq) + Br–(aq) → MnO2(s) + BrO3–(aq)
Textbook Question
Balance each redox reaction occurring in basic aqueous solution. b. Ag(s) + CN–(aq) + O2(g) → Ag(CN)2–(aq)
Textbook Question
Sketch a voltaic cell for each redox reaction. Label the anode and cathode and indicate the half-reaction that occurs at each electrode and the species present in each solution. Also indicate the direction of electron flow. a. Ni2+(aq) + Mg(s) → Ni(s) + Mg2+(aq)
Textbook Question
Calculate the standard cell potential for each of the electro- chemical cells in Problem 43.
Textbook Question
Consider the voltaic cell:
d. Indicate the direction of anion and cation flow in the salt bridge
